NL Journal of Agriculture and Biotechnology
(ISSN: 3048-9679)
Seed Oil Extraction and Analysis on the Seeds of Calophyllum apetalum Willd., an Economically Important Endemic Tree of Western Ghats
Author(s) : Raveendran M, Chinthu R V. DOI : 10.71168/NAB.02.05.127
Abstract
The composition of fats and oils is essentially the same. Both plants and animals use these compounds primarily as energy stores. A few of their constituents are vital to metabolic functions. The huge woody tree species Calophyllum apetalum is a member of the Calophyllaceae (Clusiaceae) family. The seed oil is used to cure rheumatism, leprosy, scabies and other skin conditions since it is said to have antiseptic qualities. The greenish yellow fatty oil that is produced from sun-dried kernels has a distinct odour and bitter taste. It is used as a lubricant, illuminant, to make soap and to treat leather. The seed oil is therefore extremely important. Therefore, an attempt is undertaken to extract the seed oil from C.apetalum and is a preliminary work. Keywords: Calophyllum apetalum, Seed oil extraction, Fatty acid composition, Medicinal tree species.
Introduction
Oils and fats are basically similar in composition. They are substances used by plants and animals mainly as an energy store. Some of their components are essential to metabolic processes. Many seeds are rich in fats which act as a food supply to the young seedling for example Hydnocarpus alpina is a tree species their chemical constituents found in this are chaulmoogric acid, hydnocarpic acid, apigenin, hydnocarpin, fixed oils, tannins [1] likewise the trees consist of seed oil which benefits to other organisms. The difference between an oil and a fat is that an oil is usually liquid at ambient temperature while a fat is solid [2]. The production and trade of edible oil depend heavily on oilseeds. Essential fatty acids and energy for human body come primarily from edible plant oils. Medicinal plants have immense therapeutic properties due to presence of some biological active compounds [3]. The medicinal value of plants signifies a great potential for the discovery and development of new pharmaceuticals due to its chemical substances that produce positive physiological action on human body [4].
The genus Calophyllum L. (Clusiaceae) includes about 187 species. Of these, 179 are present in the Old World (mainly in the Indo-Malaysian region) and about 8 are in the New World, from Mexico and the Caribbean to Argentina [5]. Calophyllum apetalum which is also called “Poon Spar of Travancore” belongs to the family Calophyllaceae (Clusiaceae). It is an important medicinal tree species used by traditional practitioners in Siddha, Ayurveda and folk medicines. Fruit indehiscent drupe, ovoid-subglobose, about 1-2 cm across, with thin smooth exocarp, stony endocarp, yellow/red when ripe, Seeds 1, ovoid-globose, large cotyledons, exalbuminous with oil content. C. apetalum yield edible fruits. They contain a cream – coloured kernel (average weight 2.5 g) which is eaten; it is enclosed in a hard shell (average weight 6.4g). The fruits are collected and sold. The kernels contain apetalolide and β – sitosterol- β – D - glucoside. The sun - dried kernels yield a greenish yellow fatty oil (45-50 %), having a characteristic odour and a bitter taste. Seed oil used for the treatment of leprosy and skin infections in ancient times like wise the seeds of H.alpina tree contain high level of fatty oil which is like chaulmoogra oil used extensively in the treatment of leprosy and other cutaneous diseases and also used as an illuminant [6] C. apetalum seeds dispersed by autochory, zoochory, anthropochory etc.
The tree is found along the foothills from Maharashtra and Goa southwards through the Karnataka and Kerala. It is often growing along the rivers. Species are receptive to various virus, fungi, insect, pests that affects leaves, roots and the fruits. Xanthones have been isolated from bark. Three new xanthonoids, apetalinones A-C, were isolated from the roots of C. apetalum, as well as the known compounds, calozeyloxanthone and zeyloxanthonone. The stem bark of this species yielded a new xanthonoid, apetalinone D and another known xanthonoid, tomentonone. Five known xanthones (3,8-dihydroxy-1,2-dimethoxy-, 1,3-dihydroxy-2,5-dimethoxy-, 1,5-dihydroxy-, 1,3,5-trihydroxy-2-methoxy- and 1,3,5- trihydroxyxanthone) and two flavonoids ((−)-epiafzelechin and (−)-epicatechin) were also characterized as constituents in the stem wood. Among them, apetalinone A was a novel xanthone with 1,1-dimethylallyl ether moiety, which indicated a new biosynthetic pathway including Claisen rearrangement and Diels-Alder reaction. Four new prenylated xanthonoids, apetalinones A-D, were isolated from C. apetalum in addition to five known xanthones [10]. The acetone extract of leaves of C. apetalum records the isolation and structure determination of a dipyranocoumarin α-hydroxytomentolide-A along with known friedelin, triterpenoidsapetalactone, calophyllol and inophyllum C [7].
Although C. inophyllum is a species of family Clusiaceae also have tamanu oil which has been traditionally used to treat various skin ailments, skin conditions like eczema, acne, psoriasis and dry skin. Tamanu oil possesses anti-inflammatory and antimicrobial properties, which contribute to its effectiveness in wound care and skin health. This also used to treat wounds, burns and ulcers. Studies have shown its potential in promoting wound closure and skin regeneration. C. apetalum is belonging to same family hence they also may possess more active compounds in seed oil. It is identified through seed oil extraction and further purifications.
The population of this species appears to be declining today because of numerous climate changes as well as anthropogenic activities such overharvesting of lumber, over picking of edible fruits, deforestation etc., which are the primary causes of species loss. As the data provided by IUCN Red list, this medicinally important endemic tree species C. apetalum coming under the ‘Vulnerable’ category [8]. C. apetalum has no conservation actions yet and research is needed in the areas of taxonomy, population size, distribution & trends, life history and ecology, threats and conservation planning [9]. A lack of information could be to blame for incorrect tree usage. Additionally, the seeds fall under the category of “Recalcitrant type.” These seeds during maturation having high moisture content and thus short period of viability, thus the conservation and storage of this species for longer period is problematic. A sustainable, healthy plant community can be created by phytochemical screening of the seed extract to identify compounds that may benefit humankind. This will increase our understanding of different tree species and enable us to develop effective conservation strategies. Trees are rich source of bioactive compounds continue to play a dominant role in the maintenance of human health. To increase the medical effect of natural plants, bioactive compounds must be extracted, concentrated, separated and purified.
Materials and Methods
Seed Preparation
The fresh mature seeds of Calophyllum apetalum were collected from Alappuzha, Kerala. The seeds were separated from fruits and the fruits were cracked open manually. Preparation of seeds for oil extraction involves removal of outer layers fruits to expose kernels and then drying the kernel to a desired moisture content. Five replicates of seeds were used to analyse the moisture content.
Seed Moisture Content Determination
Moisture content was determined by the difference between fresh and dry weight. For dry weight determination, the seed material was taken in a pre- weighed bottle and weighed in an electronic balance, dried in a hot air oven at High Constant Air Oven Method, 1300C for one hour [13]. For each time point, 10 replicates of a sample were made. The dry weight was recorded in every harvest, after cooling to room temperature in desiccators.
Seed Oil Extraction
Isolation of Fixed Oil
Seeds were decorticated, oven dried and powdered. 2 grams of powdered seed were filled in thimble and placed in a soxhlet extractor. The fixed oil was extracted using 250 ml n-hexane solution about 4 hours in round bottomed flask. The fixed oil collected and concentrated to 20ml using rotary evaporator and yield is noted.
The extracts were kept in airtight bottles and stored in 4⁰C for further analysis. The percentage of oil yield was calculated using equation below
TLC Profiling of Seed Oil
Thin layer chromatography of hexane extract of C. apetalum seeds were performed in pre-coated silica gel 60 F254 aluminium plate (20×20cm) with two solvent systems:
- Hexane: diethyl ether: acetic acid -8: 2: 0.2
- Hexane: diethyl ether: acetic acid -9: 3: 2
- The plates are visualized in UV chamber, iodine chamber, sprayed with 10% sulphuric acid and phosphomolybdic acid reagents and then heated at 105⁰C. The colour of the spots developed was noted.
Conversion of Extract to Fatty Acid Methyl Esters (FAME)
The extract is converted to respective methyl esters. Two drops of oil were added to 1 ml hexane and shaken for two second. Add 200 µl 2N methanolic NaOH and shaken for 10 second and placed it in a water bath at 50⁰C for 20 seconds. Add 200 µl 2N methanolic HCl and shaken. An organic and aqueous layer are formed. The organic layer is collected for further analysis.
Gas Chromatography- Mass Spectrometry (GC-MS) Analysis
Fatty Acid Methyl Esters
GC-MS analysis of FAME was carried out by 1µl injection of diluted FAME on Shimadzu Gas Chromatograph Mass Spectometer-QP2030C NX. The column used was SH-Stabliwax 1ms capillary column (30m, 0.25mm ID, O.25um).
GC-MS Operation Condition
Inject mode: split (split ratio: 50:1), Injector temperature: 240⁰C, Interface: 260⁰C, Oven temperature programmed: 60-250⁰C (3⁰C/min), Carried gas: helium 1.4 mL/min Mass spectra: electron impact (EI+) mode: 70 eV with a mass range of 50 to 550 m/z ion Ion source temperature: 240⁰C.
Results and Discussion
Moisture content measurement of an oilseed is an inevitable operation in harvesting and almost all postharvest processing such as handling, storage, milling and oil extraction. C. apetalum have high moisture content (i.e., 47.98%) when fruit at its mature stage. This is correlated with the reports of [9,10]. It was reported that the moisture content can affects the oil extraction operation. [10] investigated the effect of moisture content on oil expression from sesame seed. They reported that moisture content had the highest influence on sesame seed oil yield.
Table 1: Seed Moisture Content Determination of Calophyllum apetalum
| Sl. No | Fresh weight (g) | Dry weight (g) | Moisture content (%) ±SE |
| 1 | 4.93±0.01 | 2.64±0.01 | 46.34±0.15 |
| 2 | 4.92±0.01 | 2.62±0.01 | 47.41±0.72 |
| 3 | 4.92±0.01 | 2.64±0.03 | 46.40±0.50 |
| 4 | 4.95±0.01 | 2.64±0.01 | 46.66±0.31 |
| 5 | 4.95±0.01 | 2.53±0.02 | 47.98±0.83 |
Seed Oil Extraction
TLC Profiling of C. apetalum Seeds and Percentage of Fixed Oil
Oil content of the seeds was determined and found out to be 60 % for Alappuzha sample.
TLC profiling of C. apetalum (TLC of fixed oil in UV and Iodine chamber; fig (1) and fig (2) respectively
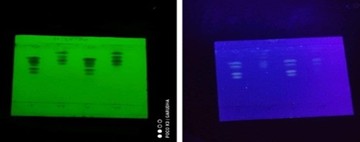 Figure 1: TLC of Fixed Oil in UV Chamber
Figure 1: TLC of Fixed Oil in UV Chamber
Solvent system: hexane, diethyl ether and acetic acid (9:3:2)
 Figure 2: TLC of Fixed Oil in Iodine Chamber
Figure 2: TLC of Fixed Oil in Iodine Chamber
GC-MS Analysis of C. apetalum Seeds
GC-MS analysis of fixed oil of C. apetalum sample from Alappuzha identified 5 components respectively. The major components in fixed oil have been identified as
- Arachidic acid
- Oleic acid
- Stearic acid
- Palmitic acid
- Myristic acid
Oleic acid and stearic acids were the predominant fatty acids in C. apetalum collected from Alappuzha. The percentage of oleic acid is higher than the stearic acids.
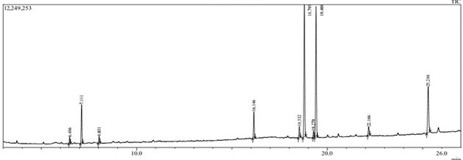 Figure 3: TLC of Fixed Oil in Iodine Chamber
Figure 3: TLC of Fixed Oil in Iodine Chamber
Table 2: Fixed Oil Constituents of C. apetalum Seed Oil
| Sl. No | Compound | Retention time (S) | Percentage of compound in the sample |
| 1 | Arachidic acid | 7.111 | 8.18% |
| 2 | Palmitic acid | 16.146 | 5.77% |
| 3 | Stearic acid | 18.795 | 31.29% |
| 4 | Oleic acid | 19.408 | 32.06% |
| 5 | Myristic acid | 22.166 | 13.76% |
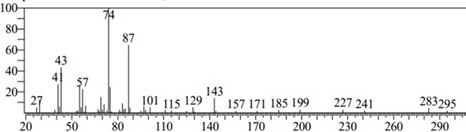 Figure 4: Chromatogram of Arachidic Acid
Figure 4: Chromatogram of Arachidic Acid
 Figure 5: Arachidic Acid
Figure 5: Arachidic Acid
Arachidic acid (C20H40O2) or icosanoic acid is a C20 straight chain saturated fatty acid which forms a minor constituent of peanut (L. arachis) and corn oils. Used as an organic thin film in the production of liquid crystals for a wide variety of technical applications. It has a role as a plant metabolite. Among the five compounds extracted, C. apetalum seed oil extract exhibits 8.18% arachidic acid, a relatively low amount. It is correlated with Theobroma grandiflorum in which the amount of arachidic acid is 7% [11] It can also be found in edible vegetable oil made from perilla seeds is called perilla oil (0-1%) [12]. And also, in Peanut oil (1.1–1.7%) [13], Corn oil (3%) [14] and Cocoa butter [15].
The least retension time is 7.111 seconds. The retension time and the amount of arachidic acid have less bearing. Arachidic acid is used as an adhesives and sealant chemicals, agricultural chemicals (non-pesticidal), finishing agents, fuel, intermediate, lubricants and lubricant additives, lubricating agent, softener and conditioner, surface active agents [16]. It is found naturally in fish and vegetable oils, and it can also be formed by the hydrogenation of arachidonic acid. Diets rich in saturated fatty acids such as arachidic acid, are known to increase serum low- density lipoproteins result in high blood cholesterol levels [17].
 Figure 6: Chromatogram of palmitic Acid
Figure 6: Chromatogram of palmitic Acid
![]() Figure 7: Palmitic Acid
Figure 7: Palmitic Acid
Palmitic acid (CH₃(CH₂)₁₄COOH) is one of the major fatty acids forming virtually all natural lipids. Both in eukary- otes and prokaryotes, C16:0 forms various lipid classes, which serve either as the lipid background of storage fats and oils, or the hydrophobic matrix of cell membranes, or the components of cuticle waxes and polymers [18].
C. apetalum seed oil through GC-MS analysis shows a concentration of 5.77% palmitic acid It is correlated with rapeseed oil 3.6% [19]. Rapeseed oil is one of the oldest known vegetable oils. There are both edible and indus- trial forms produced from rapeseed, the seed of several cultivars of the plant family Brassicaceae. Retension time is 16.146 S. Palmitic acid is the least found compound among the five compounds extracted.
Palmitic acid is known to be a primary higher fatty acid synthesized in the cell, while nearly all other fattyacids of natural lipids are the products of its further modification caused by elongation, desaturation, insertion of various functional groups, such as methyl, hydroxy, oxo, epoxy, etc.
As a saturated fatty acid, C16:0 is used by the cell for regulating its functional state by shifting the membrane fluidity under adverse environmental conditions and thus providing a necessary molecular species composition of the membrane polar lipids. Among the latter, such classes as phosphatidyl inositols, phosphatidylserines and other highly polar lipids are particularly rich in palmitic acid. In accordance, its content in plant lipids rises as they became less TLC-mobile, more difficultly extractable or tightly bounded [18].
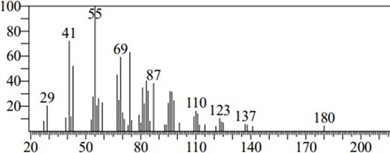 Figure 8: Chromatogram of Stearic Acid
Figure 8: Chromatogram of Stearic Acid
![]() Figure 9: Stearic Acid
Figure 9: Stearic Acid
Stearic acid, another name for octadecanoic acid CH3(CH2)16COOH, is one of the most common fatty acids. It exists as a glycerol ester in most animal and plants. Stearic acid is a prevalent fatty-acid in nature, found in many animal and vegetable fats, but is usually higher in animal fat than vegetable fat. The salts and esters of stearic acid are called stearates. Dietary sources of stearic acid include meat, poultry, fish, eggs, dairy products and foods pre- pared with fats; beef tallow, lard, butterfat, cocoa butter and shea butter are rich fat sources of stearic acid [16].
The GC- MS analysis of C. apetalum shows 31.29% stearic acid it is correlated with the stearic acid present in the Cocoa butter (34%) (16,13) stearic acid is more abundant in animal fat up to 33% in beef liver [13] similarly close to that much amount of stearic acid is present in C. apetalum. Thus, its value is significant. The retension time is 18.795 S.
In general, the applications of stearic acid exploit its bifunctional character, with a polar head group that can be attached to metal cations and a nonpolar chain that confers solubility in organic solvents. The combination leads to uses as a surfactant and softening agent. Stearic acid undergoes the typical reactions of saturated carboxylic acids, a notable one being reduction to stearyl alcohol and esterification with a range of alcohols [16].
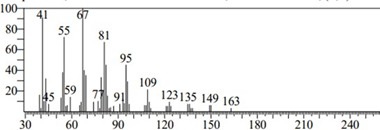 Figure 10: Chromatogram of Oleic Acid
Figure 10: Chromatogram of Oleic Acid
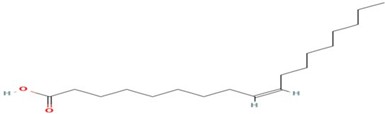 Figure 11: Oleic Acid
Figure 11: Oleic Acid
Oleic acid (C18H34O2) is a monounsaturated omega-9 fatty acid recognized as cis-9-octadecenoic acid. Oleic acid has many beneficial effects on human health. One of the main dietary sources of oleic acid is olive oil. Numerous epidemiological, clinical and experimental research have shown that consumption of Mediterranean diet rich in olive oil has a profound influence on several health outcomes, including obesity, metabolic syndrome, cardiovascular disease and diabetes mellitus [20]. The GC- MS analysis of C. apetalum shows 32.06% oleic acid. It is very close to the amount present in peanut oil. In which it is 36–67% [21]. Oleic acid concentration is high among the five compounds extracted. The retention time is 19.408 S. Besides the favourable effects of oleic acid intake in adults, it is also essential in infants and children nutrition. Oleic acid is a component of tissues and membranes and a major fatty acid (FA) component of brain myelin phospholipid, which is mainly formed during the two years after birth [22]. Since oleic acid is rapidly deposited during myelination, its proportion in brain total lipids increases with progressive central nervous system myelination [23]. Thus, intake of oleic acid is of a great importance in post-natal life. Oleic acid C18: 1 (39.1–50%) is the dominating fatty acid in Calophyllum calaba [24].
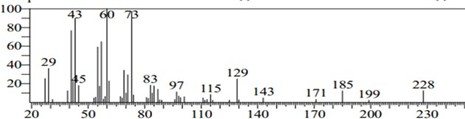 Figure 12: Chromatogram of Myristic Acid
Figure 12: Chromatogram of Myristic Acid
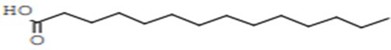 Figure 13: Myristic Acid
Figure 13: Myristic Acid
Myristic acid (CH3(CH2)12COOH) is a common saturated fatty acid. The salts and esters are called myristates or tetradecanoates. It was first isolated from nutmeg [25]. Myristic acid (C14:0) usually accounts for small amounts (less than 1 wt%) of total fatty acids in animal tissues but is abundant in milk fat (7–12 wt% of total fatty acid [26]. Besides nutmeg, myristic acid is found in palm kernel oil, coconut oil, butterfat, 8–14% of bovine milk and 8.6% of breast milk as well as being a minor component of many other animal fats.The GC- MS analysis of C. apetalum shows 13.76% myristic acid, close to the amount present in the bovine milk (8–14%) [13]. Besides nutmeg, myristic acid is found in palm kernel oil, coconut oil, butterfat, 8.6% of breast milk as well as being a minor component of many other animal fats. Myristic acid shows the highest retention time 22.16 S. All the chemical compounds analyzed through GC-MS. Oleic acid (32.06%) and stearic acid (31.29%) are the major constituents of the C. apetalum seed oil. Palmitic acid (5.77%) is the least found compound, while arachidic acid (8.18%) and myristic acid (13.76%) are also found in relatively low amount. Myristic acid shows the highest retention time (22.16 S) and arachidic acid shows the lowest retention time (7.111 S). The chemical composition of C. apetalum seed oil shows similarities with the closely related species C. inophyllum. It has similar compounds like oleic acid (39.1 ± 1.4%), palmitic acid (13.7 ± 0.8%) (28) with small difference in percentage composition compared to C. apetalum. However other compounds like stearic acid (14.3 ± 0.8%), myristic acid (<0.1%), ara- chidic acid (0.6%) [29] is less dominant in C. inophyllum compared to C. apetalum. The fatty acid profile of seed oil makes it a suitable candidate for biodiesel production. Presence of high amounts of unsaturated fatty acids makes C. inophyllum candidate for biodiesel production [30]. However unsaturated fatty acid composition of C. apetalum is not par with that of C. inophyllum and small thus total seed oil content compared to the other makes it probably not suitable for large sale biodiesel production. However, the fatty acid profile explains its use of lamp oil in the traditional systems.
Conclusion
Calophyllum apetalum seed oil extract contains fixed oils such as arachidic acid, palmitic acid, stearic acid, oleic acid and myristic acid. In which the oleic acid found to be more than other fatty oils. This work must be used as preliminary data for the extraction, quantification and purification of seed and seed oil to produce medicinal products economically.
References
1. Chinthu RV, Raveendran M, seed germination and restoration studies of Hydnocarpus alpina wight; an endemic medicinally important tree species of Western Ghats.2023; IJSDR vol 8, issue 5.
2. Head SW, Swetman AA, Hammonds TW, Gordon A, Southwell KH, Harris RV, Small Scale Vegetable Oil Extraction.1995; Natural Resources lnsdtiute, Overseas Development Administration. Hobbs the Printers, Totton, Hampshire.
3. Sayantani Chanda and Ramachandra TV, A review on some Therapeutic aspects of Phytochemicals present in Medicinal plants, International Journal of Pharmacy and Life sciences.2019; 10(1):6052-6058.
4. Phytochemical and Pharmacological Properties of Medicinal Plants from Uzbekisthan: A Review. Dilfuza Egamberdieva. Vol.5, issue 2, vol 5 issues 1-4, Journal of Medicinally Active Compounds.
5. Chinthu RV, Raveendran PB, Raveendran M A review on the genus Calophyllum L. (Clusiaceae): a potential medicinal tree species. (2022); Plant Science Today https://doi.org/10.14719/pst.1818.
6. Chinthu RV, Raveendran M. Effect of desiccation damage on the seed viability of Hydnocarpus alpina Wight of Western Ghats. Plant Science Today. 2023; 10(1):15–21. https://doi.org/10.14719/pst.1655.
7. Ganeshaiah KN, Kailash BR. ATREE, Bangalore, India. Royal Norwegian Embassy grants. Indian Bioresource Information Network (IBIN), Department of Biotechnology, New Delhi.
8. Chinthu RV, Raveendran M and Raveendran PB, A Review on Calophyllum apetalum Willd., an Endemic Medicinal Tree of Western Ghats. Research Journal of Pharmaceutical, Biological and Chemical Sciences.2023;14(6).
9. Chinthu RV, Raveendran M and Raveendran PB Phenological studies of Calophyllum apetalum Willd., an economically important medicinal tree of Western Ghats. J. Indian bot. Soc., (2024). Doi: 10.61289/jibs2025.02.22.0342.
10. MunekazuIinuma, Tetsuro Ito, Hideki Tosa, Toshiyuki Tanaka, Ryoko Miyake, Veliah Chelladurai. Phytochemistry. 1997; 46(8):1423-1429
11. International Seed Testing Agency (1985).
12. Olayanju TMA, Akinoso R and Oresanya M O. Effect of wormshaft speed, moisture content and variety on oilrecovery from expelled beniseed. Agricultural Engineering International: the CIGR E journal.2006; Vol. 8, Manuscript FP06008.
13. Akinoso R, Igbeka J, and Olayanju T. Process optimization of oil expression from sesame seed (sesamum indicumlinn.). Agricultural Engineering International: the CIGR Ejournal.2006; Vol. 8, Manuscript FP06011
14. Cohen K de O & Jackix M de NH. “Caracteristicas químicas e fisica da gordura de cupuaçu e da manteiga de cacau” (PDF). Document / Embrapa Cerrados (in Portuguese.2009; (269): 1–22.
15. Shin Hyo-Sun. Lipid Composition and Nutritional and Physiological Roles of Perilla Seed and its Oil”. In Yu, He-Ci; Kosuna, Kenichi; Haga, Megumi (eds.). Perilla: The Genus Perilla. London: CRC Press. 1997”9. p. 93. doi:10.1201/9781439822715.
16. Beare-Rogers J, Dieffenbacher, A, Holm, JV “Lexicon of lipid nutrition (IUPAC Technical Report)”. Pure and Applied Chemistry. 2001; 73 (4):685–744. doi:10.1351/pac200173040685.
17. U.S. Department of Agriculture, Agricultural Research Service. 2007. USDA National Nutrient Database for Standard Reference, Release 20.
18. USDA Nutrient database; 2015
19. PubChem, US National Library of Medicine. 2023.
20. https://www.scbt.com/p/arachidic-acid-506-30-9
21. Sidorov, Roman & Zhukov, Anatoly & Pchelkin V & Tsydendambaev, Vladimir. 2014. Palmitic Acid in Higher Plant Lipids.
22. Nelson, Gary J. Health Effects of Dietary Fatty Acids. American Oil Chemists’ Society. 1991; pp. 84-86.
23. Perez-Martinez P, Garcia-Rios A, Delgado-Lista J, Perez-Jimenez F, Lopez-Miranda J: Mediterranean dietrich in olive oil and obesity, metabolic syndrome anddiabetes mellitus. Curr Pharm Des. 2011; 17(8):769-77.
24. Crane S, Aurore G, Joseph H, Mouloungui Z, Bourgeois P. Composition of fatty acids triacylglycerols and unsaponifiable matter in Calophyllum calaba L. oil from Guadeloupe. Phytochemistry. 2005; 66 (15):1825–1831.
25. Moore, K. M.; Knauft, DA. “The Inheritance of High Oleic Acid in Peanut”. The Journal of Heredity. 1989;80 (3): 252–3.
26. Sahin N, Akoh CC, Karaali A: Human milk fat substitute containing omega-3 fatty acids. J Agric Food Chem. 2006; 54(10):3717-22.
27. Rioux FM, Innis SM: Oleic acid (18:1) in plasma, liver and brain myelin lipid of piglets fed from birth with formulas differing in 18:1 content. J Nutr. 1992; 122(7):1521-8.
28. Playfair, Lyon. On a new fat acid in the butter of nutmegs”. Philosophical Magazine. Series 3. 2009”XX; 18(115):102–113.
29. Jensen RG, Ferris AM, Lammi-Keefe CJ, Henderson RA. Lipids of bovine and human milks: a comparison. J Dairy Sci.1990;73: 223–240.
30. Atabani AE, Cesar ADS, Calophyllum inophyllum L. A prospective non-edible biodiesel feedstock. Study of biodiesel production properties, fatty acid composition, blending and engine performance. Renew. Sustain. Energy Rev. 2014; 37:644–655.
This article licensed under the Creative Commons Attribution 4.0 International License CC-BY 4.0., which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are properly credited.
