NL Journal of Agriculture and Biotechnology
(ISSN: 3048-9679)
Morphometric, Meristic and Growth Patterns of Sphyraena Afra from the Escravos Estuary
Author(s) : Ochuko Joshua Eriegha, Chinyere Mirabel Anumudu, Victor Oscar Eyo, Aminu Abubakar Garba. DOI : 10.71168/NAB.02.05.126
Abstract
This study presents a biological assessment of Sphyraena afra from the Escravos Estuary, focusing on morphometric and meristic traits, length, weight relationship, and condition factor. A total of 189 specimens were collected from artisanal landings between May 2023 and April 2024. Sixteen morphometric and five meristic parameters were measured to evaluate body proportions and fin structure. Total length ranged from 12.40 to 42.00 cm, and body weight from 10.00 to 292.00 g. Notable variation was observed in pelvic fin rays (range: 9-15) and pectoral fin rays (range: 5-6), while dorsal spines were consistent across individuals. Principal component analysis revealed that the first two components explained 100% of the total variation. PC1 was dominated by morphometric traits associated with body size, while PC2 was largely influenced by meristic characters. The length-weight relationship followed the equation: 𝑊 = 0.0043𝑇𝐿2.9547 with a high correlation coefficient (R² = 0.986), indicating near-isometric growth. The mean condition factor (K = 0.3740 ± 0.0048) suggests moderate physiological condition and supports evidence of slow but steady growth. Although morphometric traits were size-dependent, the observed meristic variation may reflect environmental or developmental influences. These findings provide a useful baseline for future ecological assessments, monitoring, and stock evaluation of S. afra in Nigerian estuarine waters. Further studies integrating genetics, ecology, and broader spatial data are recommended to support sustainable management of this species. Keywords: Morphological analysis, Fish growth pattern, Stock assessment, Escravos estuary.
Introduction
Accurate identification and species characterization form the foundation of effective fish conservation and sustainable fisheries management. Among the various methods used, morphological features remain a vital source of taxonomic and ecological information in fish biology [1]. In recent decades, morphometric analysis has gained wide application as a powerful method for detecting subtle shape differences that are not influenced by size. This makes it highly effective for evaluating population structures and characterizing distinct stocks or strains within a species. Although molecular techniques have advanced considerably, providing direct insights into genetic variation, the continued relevance of morphometric and meristic approaches cannot be overstated [2,3]. The examination of body proportions, fin arrangements, scale counts, and other physical attributes continues to provide essential data for fisheries management, conservation biology, and comparative evolutionary studies.
Nigeria possesses vast and diverse fisheries resources spread across marine, coastal, estuarine, and inland eco- systems. The fisheries sector plays a vital role in the national economy, providing food, employment, and income for millions of people [4]. Estuarine environments, in particular, are biologically productive zones that serve as nursery and feeding grounds for numerous commercially important species.
However, these ecosystems face growing pressures from overfishing, pollution, habitat alteration, and climate- related impacts. Despite the ecological and economic significance of Nigeria’s fisheries, many exploited fish species remain poorly studied, especially in terms of their morphological diversity [5]. Comprehensive biological studies are urgently needed to support evidence-based management and ensure long-term sustainability of fish stocks in the country’s coastal and estuarine waters.
Sphyraena afra, commonly referred to as the Guinean barracuda, is a predatory fish belonging to the family Sphy- raenidae [6]. It is widely distributed along the eastern Atlantic coast of West Africa, where it inhabits shallow marine and estuarine environments. The species is characterized by a streamlined body, pointed snout, large mouth with conical teeth, and a forked caudal fin. Sphyraena afra is increasingly exploited by artisanal fishers in Nigeria, contributing to the food supply and local economies of coastal communities [7]. Despite its growing presence in fisheries landings, scientific data on its morphological features in Nigerian waters remain scarce. Understanding its morphometric and meristic traits is particularly important in estuarine systems like the Escravos Estuary, where environmental variability and human activities may influence phenotypic expression through mechanisms such as phenotypic plasticity or local adaptation.
The Escravos Estuary, situated in the western Niger Delta region, is a dynamic transitional ecosystem influenced by both marine and freshwater inputs. It is subject to varying salinity regimes, sedimentation patterns, and anthropogenic disturbances including oil exploration and industrial discharge [8]. These environmental gradients can exert selective pressures on aquatic organisms, potentially leading to morphological divergence within spe- cies. Given the ecological complexity of this habitat and the limited biological data available for S. afra in Nigerian waters, a detailed morphological and growth assessment is both timely and necessary. This study investigates the morphometric and meristic features, length–weight relationship, and condition factor of S. afra from the Escravos Estuary. While morphometric traits are expected to reflect individual growth variation, meristic characteristics and growth indicators may offer insight into environmental influence or stock condition. The findings aim to enhance biological understanding of the species and support future efforts in estuarine fisheries management and biodiversity monitoring.
Materials and Methods
Study Area
The study was conducted in the Okerenkoko section of the Escravos Estuary, located in the western Niger Delta region of southern Nigeria. This estuarine system forms part of a complex network of tidal creeks, mangrove swamps, and coastal sandbanks that drain into the Bight of Benin. The Escravos Estuary is ecologically diverse and supports a wide range of aquatic species, making it an important fishing ground for local communities [9]. The area is dominated by extensive mangrove vegetation and brackish water conditions, with salinity levels rang- ing from 14 to 31 parts per thousand depending on tidal influence and seasonal variation. The estuary experi- ences a mix of freshwater inflows and tidal surges from the Atlantic Ocean, creating a dynamic environment that supports both marine and freshwater species [10].
The primary livelihood of residents in the Okerenkoko community includes artisanal fishing and fish processing, alongside the construction and use of traditional fishing gears such as basket nets, traps, gill nets, drag nets, and hook and line systems. Petty trading also contributes to the local economy. In addition to subsistence and com- mercial fishing, the region hosts significant oil exploration and production activities, which introduce multiple anthropogenic pressures on the estuarine ecosystem [11]. Figure 1 presents the map of the study area, highlight- ing the Okerenkoko sampling location within the broader Escravos Estuary.
 Figure 1: Map showing sample stations along the Escravos Estuary (Source [8])
Figure 1: Map showing sample stations along the Escravos Estuary (Source [8])
Sample Collection
A total of one hundred and eighty-nine (189) specimens of S. afra were randomly collected from prominent fish landing sites in Okerenkoko between May 2023 and April 2024. The samples were obtained from the catches of artisanal fisherfolk using traditional fishing gears such as gill nets and dip nets. Immediately after landing, fresh specimens were transported to the laboratory of the Department of Fisheries and Aquaculture, Faculty of Environmental Management, Nigeria Maritime University, Okerenkoko, for species identification and biometric analysis. Identification was conducted using standard taxonomic keys and morphological descriptions provided by Ballen [12]. All samples were handled carefully to preserve the integrity of morphological features necessary for accurate measurement and analysis.
Measurement of morphometric parameters of Sphyraena afra
Measurement for their length and weight were determined using a meter rule and a digital scale (OHAUS Cor- poration, USA), respectively. The following body characteristics and morphometric were also be measured: Total Length (TL), Standard Long (SL), Fork Length (FL), First Dorsal Fin Base (FDF), Second Dorsal Fin Base (SDF), Caudal Peduncle Depth (CPD), Caudal Peduncle Length (CPL), Gape Width (GW), Per Orbital Length (POL), Eye Di- ameter (ED), Post Orbital Length (PSOL), Head Length (HL), Pelvic Fin Length (PFL), Pectoral Fin Length (PCFL), and Anal Fin Length (AFL).
Determination of Meristic Features
Meristic features of S. afra were determined through direct visual inspection of each specimen under appropri- ate lighting. During examination, each fish was positioned with the head facing to the left to ensure uniformity in counting. A single count was recorded when two fin rays originated from a common base, in accordance with standard meristic counting procedures. The meristic characters assessed included the number of dorsal spines, dorsal fin rays, anal fin rays, pelvic fin rays, and pectoral fin rays. These features were carefully counted and docu- mented for each specimen, providing essential data for species identification and morphological characterization.
Determination of length-weight Relationship
The relationship between fish length and weight was expressed using Ricker’s equation [13] after data were log-transformed to address non-normality.
W = a.Lb
Where,
L = total length (cm)
W = body weight (g)
a = constant and,
b = slope (fish growth rate).
Using least squares linear regression, the constants (a and b) were derived from the log-transformed length and weight values (log W = log a + b log L).
Condition Factor (K)
The condition factor (K values) was calculated using Fulton’s condition factor formula [14].
K = 100W/L3
Where:
K = Condition factor,
W = Weight of fish (g) and
L = length of fish (cm).
Statistical Analysis
Morphometric and meristic characters were reported as mean and ranges. Statistical analysis was conducted using the SPSS (Version 20). Additionally, morphometric and meristic data underwent principal component analysis using Origin Pro (version 2022) to identify patterns in correlated variables.
Results and Discussions
Morphometric Characteristics
The mean and standard error of the mean of 16 morphometric measurements of S. afra from the Escravos Estuary are shown in Table 1. The result revealed that the total length of the specimens ranged from 12.40 to 42.00 cm, with a mean of 25.02 ± 0.85 cm.
Fork length ranged from 2.10 to 40.00 cm (mean: 21.85 ± 0.87 cm), while standard length varied from 10.50 to 37.00 cm (mean: 20.59 ± 0.71 cm). The body weight of the fish ranged from 10.00 to 292.00 g, with a mean value of 69.53 ± 8.02 g. Additional traits such as head length (mean: 6.65 ± 0.21 cm), caudal peduncle length (3.08 ± 0.13 cm), gape width (3.13 ± 0.10 cm), and eye diameter (1.08 ± 0.02 cm) showed moderate variability across individuals.
The observed total length range for S. afra in this study was slightly higher than the length range reported for Sphyraena chrysotaenia (14.0 to 34.5 cm) in the northeastern Mediterranean [15]. This difference may be linked to factors such as phenotypic plasticity, local adaptation, or geographic separation, as suggested by Jacob [16]. Environmental influences are known to shape morphometric traits, as phenotypic variation can result from both genetic and environmental factors [17]. Krabbenhoft [18] identified water clarity, depth, current velocity, food availability, and physical habitat complexity as environmental variables that can significantly influence fish morphology.
Table 1: Mean and standard error for morphometric of Sphyeaena afra from the Escravos Estuary
| Traits | Mean ± SE | Range |
| Weight (WT) | 69.53 ± 8.02 | 10.00-292.00 |
| Total length (TL) | 25.02 ± 0.85 | 12.40-42.00 |
| Standard length (SL) | 20.59 ± 0.71 | 10.50-37.00 |
| Fork length (FL) | 21.85 ± 0.87 | 2.10-40.00 |
| First dorsal fin base(FDF) | 2.28 ± 0.08 | 1.00-4.20 |
| Second Dorsal fin base(SDF) | 2.08 ± 0.07 | 0.90-3.60 |
| Caudal peduncle Depth (CPD) | 1.32 ± 0.05 | 0.60-2.40 |
| Caudal Peduncle length (CPL) | 3.08 ± 0.13 | 1.00-5.50 |
| Gape width (GW) | 3.13 ± 0.10 | 1.60-5.20 |
| Pre- orbital length (POL) | 3.10 ± 0.11 | 0.50-5.00 |
| Eye diameter (ED) | 1.08 ± 0.02 | 0.70-1.40 |
| Post-orbital length (PSOL) | 2.35 ± 0.09 | 1.20-4.60 |
| Head length (HL) | 6.65 ± 0.21 | 3.50-11.00 |
| Pelvic fin length (PFL) | 2.31 ± 0.11 | 0.50-4.10 |
| Pectoral fin length (PCFL) | 2.07 ± 0.07 | 0.90-3.50 |
| Anal length (AL) | 2.91 ± 0.10 | 1.40-5.00 |
Meristic Characteristics of S. afra
The mean and standard error of the mean of 5 meristic count made on S. afra from the Escravos Estuary are shown in Table 2. The number of dorsal spines was consistent across all specimens examined, with a count of five, showing no variation (mean: 5.00 ± 0.00). This stability indicates that dorsal spine count may be a geneti- cally fixed trait within this population. In contrast, the number of pelvic fin rays showed the widest range among the meristic features, varying between 9 and 15 rays (mean: 11.88 ± 0.13). The pectoral fin rays ranged between 5 and 6, with a mean value of 5.78 ± 0.11. Dorsal rays ranged from 8 to 10 (mean: 8.94 ± 0.11), while anal fin rays varied from 7 to 9 (mean: 8.31 ± 0.11). This level of variation in ray counts is typical of many teleosts and may reflect developmental plasticity or environmental influence during early life stages. The variation observed in meristic traits, particularly fin rays, can serve as useful taxonomic and ecological markers when comparing populations from different habitats or regions.
When compared with the results of S. afra collected from Lagos Lagoon [19], some notable differences emerge. In that study, the number of first dorsal spines, second dorsal fin rays, and pelvic fin rays were constant at 5, 9, and 6, respectively, while the anal fin rays ranged from 8 to 10 (mean: 8.55 ± 0.012). Pectoral fin rays in their study ranged from 10 to 12 (mean: 11.82 ± 0.013), which is considerably higher than the range of 5 to 6 observed in the current study. This suggests the possibility of regional variation or stock differentiation between the Escravos and Lagos populations. The discrepancies observed in pelvic and pectoral fin ray counts between the two studies may be attributed to genetic variation, developmental plasticity, or environmental influences such as salinity, habitat complexity, or temperature differences between the estuaries. Swain and Foote [17] noted that variation in meristic counts may not be entirely genetic but could also be environmentally induced.
Such differences are important in fisheries biology and taxonomy, as they may signal localized adaptations or even subtle population structuring across geographically separated habitats. The relatively high variability observed in pelvic fin rays in the current study (ranging from 9 to 15) contrasts sharply with the fixed value of 6 reported by Ayo-Olalusi [7], further supporting the possibility of environmental or developmental influence. These findings highlight the need for broader geographic sampling and possibly genetic analysis to determine whether these differences reflect phenotypic plasticity or underlying population divergence.
Table 2: Mean and standard error for meristic of Sphyraena afra from the Escravos Estuary
| Traits | Mean ± SE | Range |
| Number of dorsal spines | 5.00 ± 0.00 | 5.00-5.00 |
| Number of dorsal rays | 8.94 ± 0.11 | 8.00-10.00 |
| Number of anal fin rays | 8.31 ± 0.11 | 7.00-9.00 |
| Number of pelvic fin rays | 11.88 ± 0.13 | 9.00-15.00 |
| Number of pectoral fin rays | 5.78 ± 0.11 | 5.00-6.00 |
Principal Component Analysis of Morphometric and Meristic Traits
The results of the principal component analysis (PCA) of the morphometric and meristic features of S. afra from the Escravos estuary is presented in Figure 2. The analysis revealed that the first two principal components (PC1 and PC2) accounted for a substantial portion of the total variation observed in the dataset, indicating that a few key variables drive much of the morphological differentiation within the population. The first principal compo- nent (PC1), which explained the largest proportion of variation, was strongly influenced by traits such as stand- ard length, pre-dorsal length, body depth, and head length. These features are closely linked to the general body size and swimming capability of the fish. The strong loading of standard length and pre-dorsal length on PC1 sug- gests that overall body elongation and the positioning of the dorsal fin are major contributors to morphological variability. This may reflect individual differences in growth stages or adaptations to hydrodynamic conditions within the estuary. Longer and deeper-bodied individuals may be better adapted for maneuverability in shallow, vegetated, or current-influenced environments, typical of estuarine systems like Escravos [19].
The second principal component (PC2) was influenced more by meristic features such as the number of dorsal fin rays, anal fin rays, and pectoral fin rays. These traits, although generally stable, can exhibit variation due to developmental or environmental factors. The prominence of these fin ray counts on PC2 suggests that even slight differences in fin structure may play a role in differentiating individuals, possibly affecting swimming efficiency or stability in fluctuating salinity and turbidity conditions. Interestingly, while some traits such as pelvic fin rays and caudal peduncle depth contributed minimally to either component, their low variability may indicate a high level of conservation in these structures within the population, possibly due to their critical functional roles in propulsion and balance [20]. The distribution of individuals in the PCA plot did not reveal distinct groupings, suggesting a relatively homogeneous population structure. However, the observed spread along the principal components reflects a continuum of morphological variation, potentially influenced by environmental gradients, ontogenetic shifts, or subtle habitat preferences within the estuary.
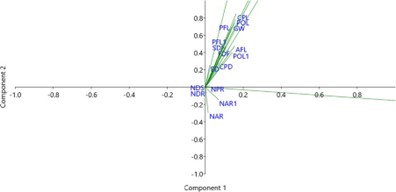
Figure 2: Principal component analysis of the morphometric and meristic parameters of s. afra from the Escravos estuary
Length-Weight Relationship
The length - weight regression graph of S. afra from the Escravos estuary can be described by the following equation: W = 0.0043TL2.9547, R² = 0.986. The ‘b’ values estimated for S. afra using the regression model was not significantly different from ‘3’ at 95% confidence limit. The results also revealed that fit index or modelling efficiency was high for both equations.
The length-weight relationship value of 2.95 for S. afra implies near-isometric growth, indicating that the fish maintains proportional increases in length and weight as it grows. The computed slope in this study (2.95) was slightly higher than values reported for S. afra from Lagos Lagoon, where b ranged from 2.72 to 2.85 [7]. The variation may be attributed to differences in sample size, size classes, environmental conditions, or seasonal effects. Length-weight relationships are known to be influenced by a variety of intrinsic and extrinsic factors including sex, age, temperature, feeding intensity, reproductive stage, and habitat [21,22,23].
Condition Factor
The condition factor (K) provides an index of the general wellbeing or robustness of fish and is often used to evaluate habitat suitability and health status [24]. In this study, the condition factor of Sphyraena afra from the Escravos Estuary ranged from 0.31 to 0.52, with a mean value of 0.3740 ± 0.0048. According to Bagenal and Tesch [25], higher condition factor values typically suggest that fish are in better physiological condition, while lower values may indicate environmental stress or poor feeding conditions. The average condition factor recorded in this study reflects relatively slow growth but remains comparable with the condition factor of Sphyraena chryso- taenia (K = 0.39) reported in the Egyptian Mediterranean [26]. The value observed here was higher than that reported by Ayo-Olalusi [7] for S. afra from Lagos Lagoon (K = 0.1), indicating better nutritional or environmental conditions in the Escravos Estuary. It also exceeded the K value of Sphyraena obtusata (K = 0.07) recorded off the coast of India [27].
The relatively low but stable condition factor values reported in this study may be characteristic of species with slow growth rates and large asymptotic sizes. Indeed, Sphyraena afra has been shown to possess a large asymp- totic size and high growth performance index when compared with other Sphyraena species [28]. Its slow growth and long lifespan (up to 30 years) may also make it vulnerable to overfishing, a trait commonly seen in long-lived species [29]. King [30] noted that industrial activity and noise from outboard engines could influence fish growth patterns, while Sparre and Venema [31] emphasized that growth parameters may vary even within the same species depending on environmental and ecological conditions. Furthermore, condition factor can also fluctuate based on nutritional status, life history events such as spawning or metamorphosis, and seasonal changes [32]. These factors collectively underscore the importance of interpreting condition factor values within the broader ecological and physiological context of the fish population.
Conclusion
This study provides a comprehensive description of the morphometric, meristic, and growth patterns of Sphyraena afra from the Escravos Estuary. The morphometric traits showed a wide range in body sizes, largely reflecting individual growth variation. Meristic counts, particularly in pelvic and pectoral fin rays, showed some variability that may indicate environmental or developmental influence.
The length-weight relationship indicated near-isometric growth, reflecting proportional increases in body length and weight, while the condition factor values pointed to a population with moderate physiological status. These findings collectively underscore the ecological relevance of S. afra and its potential vulnerability to environmental pressures and fishing activities due to its slow growth pattern. These results offer a valuable biological baseline for S. afra in a key Nigerian estuarine system. Although no clear evidence of population-level divergence was found, the observed traits are relevant for understanding the ecological adaptability and growth potential of this fish species. Future research incorporating genetic tools, reproductive data, and wider spatial sampling is recommended to further clarify stock structure and support sustainable management of this important species.
References
1. Eriegha OJ, Ekelemu JK, Nwachi OF. Comparative Morphology of Ethmalosa fimbriata (Bowdich, 1825) From Three Estuaries Adjoining the Gulf of Guinea, Nigeria. ILMU KELAUTAN: Indonesian Journal of Marine Sciences. 2024; 29(2):211-221.
2. Ewhuwhe-Ezo J, Eriegha OJ, Eyo, VO. Morphological Characterization and DNA Barcoding of Lutjanus agennes (African Red Snapper) from Escravos and Qua Iboe Estuaries. International Journal of Maritime and Interdis ciplinary Research. 2024; 6(2): 18 -31.
3. Maqbul I, Alina DN, Salma U, Agung MU. Morphological and genetical approaches to examine the threadfin bream collected from Muara Baru modern fish market, Jakarta. Fisheries and Aquatic Sciences. 2025; 28(6):425-38.
4. Ateme ME. Developing marine and coastal resources in Nigeria: Prospects and challenges. Maritime Technology and Research. 2021; 3(4):335-47.
5. Kazeem KO, Olanrewaju NA. Eco-morphological diversity of fish fauna in a tropical man-made lake, Southwestern Nigeria. Aceh Journal of Animal Science. 2025;10(1):1-0.
6. Park KN, Song YS, Kim JK. Molecular Phylogeny and Taxonomic Review of the Family Sphyraenidae from Korea. Korean Journal of Ichthyology. 2024; 36(4):307-18.
7. Ayo-Olalusi CI, Abeke Ayoade A. Population parameters of barracuda, Sphyraena afra (Family: Sphyraenidae) from coastal waters of Lagos State, Nigeria. Zoology and Ecology. 2018; 28(4):376-83.
8. Eriegha OJ, Ekelemu JK, Nwachi OF, Eyo VO. Histopathology of Selected Economically Important Fish Species from the Escravos Estuary, Nigeria: A Baseline Study for Environmental Monitoring. International Journal of Innovative Studies in Aquatic Biology and Fisheries. 2024; (9):1-8.
9. Eyo VO, Ajang R, Ewutanure SJ, Eriegha OJ, Eze F, Udobong BE. Mineral composition of important shell fish species from the Escravos River Estuary, Delta State, Nigeria and their nutritional significance. J. Adv. Food. Sci. Technol.2023;23;10:10-9.
10. Ogbeibu AE, Oribhabor BJ. The Niger Delta mangrove ecosystem and its conservation challenges. Mangrove biology, eco system, and conservation. 2023; 22;19.
11. Binyotubo TE, Eyo VO, Eriegha OJ, Eze, F. Status and Constraints of Artisanal Fisher’s in Escravos Estuary around Okeren koko and Kurutie Axis, Niger Delta, Nigeria. Advances in Multidisciplinary and Scientific Research. 2022; 1: 51–62.
12. Ballen GA. Nomenclature of the Sphyraenidae (Teleostei: Carangaria): A synthesis of fossil-and extant-based classifiction systems. Zootaxa. 2019 Oct 16;4686(3):397-408.
13. Ricker WE. Two mechanisms that make it impossible to maintain peak-period yields from stocks of Pacific salmon and other fishes. Journal of the Fisheries Board of Canada. 1973; 30(9):1275-86.
14. Pauly D. Fish population dynamics in tropical waters: a manual for use with programmable calculators. WorldFish; 1984.
15. Erguden D,Ozdemir O.Age, Growth and mortality rate of yellowstripe barracuda,Sphyraena chrysotaenia Klunzinger1884 living in the Northeastern Mediterranean. Thalassas: An International Journal of Marine Sciences.2022;38(2):1165-74.
16. Jacob OO, Solomon SG, Cheikyula JO. Morphometric and Meristic Characterization of Common Carp, Cyprinus Carpio Strains Sourced from Jos, Bauchi and Ibadan, Nigeria. Nigerian Annals of Pure and Applied Sciences. 2018; 1:82-7.
17. Swain DP, Foote CJ. Stocks and chameleons: the use of phenotypic variation in stock identification. Fisheries Research.1999;43(1-3):113-28.
18. Krabbenhoft TJ, Collyer ML, Quattro JM. Differing evolutionary patterns underlie convergence on elongate morphology in endemic fishes of Lake Waccamaw, North Carolina. Biological Journal of the Linnean Society. 2009; 98(3):636-45.
19. Teichert N, Pasquaud S, Borja A, Chust G, Uriarte A, Lepage M. Living under stressful conditions: Fish life history strategies across environmental gradients in estuaries. Estuarine, Coastal and Shelf Science. 2017; 188:18-26.
20. Aiello BR, Hardy AR, Cherian C, Olsen AM, Ahn SE, Hale ME, Westneat MW. The relationship between pectoral fin ray stiffness and swimming behavior in Labridae: insights into design, performance and ecology. Journal of Experimental Biology.2018; 221(1):jeb163360.
21. Abbasi K, Mouludi-Saleh A, Eagderi S, Sarpanah A. Length-weight relationship and condition factor of eight species of the genera Capoeta, Garra, Chondrostoma, Schizothorax and Paraschistura from Iranian inland waters. Iranian Journal of Ichthyology. 2019; 6(4):264-70.
22. Mehmood S, Ahmed I, Ali MN. Length-weight relationship, morphometric and meristic controlling elements of three freshwater fish species inhabiting North Western Himalaya. Egyptian Journal of Aquatic Biology & Fisheries. 2021; 25(6).
23. Eriegha OJ, Eyo VO. Length-Weight Relationship and Condition Factor of Ethmalosa fimbriata(Bowdich, 1825)from the Escravos Estuary, Delta State, Nigeria.J of Aquac Fisheries. 2023; 7:1-5.
24. Li Y, Feng M, Huang L, Zhang P, Wang H, Zhang J, Tian Y, Xu J. Weight–length relationship analysis revealing the impacts of multiple factors on body shape of fish in China. Fishes. 2023 May 19; 8(5):269.
25. Bagenal TB, Tesch AT. Conditions and growth patterns in fresh water habitats. Methods for assessment of fish production in freshwaters. 1978; 101-136.
26. Allam SM, Faltas SN, Ragheb EV. Age and growth of barracudas in the Egyptian Mediterranean waters. Egyptian Journal of Aquatic Research. 2004; 30(2):281-289.
27. Najmudeen TM, Seetha PK, Zacharia PU. Fishery and population dynamics of the obtuse barracuda Sphyraena obtusata (Cuvier) landed by trawlers at Cochin, south-west coast of India. Indian Journal of Fisheries. 2015; 62(2):14-18.
28. ElGanainy A, Amin A, Ali A, Osman H. Age and growth of two barracuda species Sphyraena chrysotaenia and S. flavicauda (Family: Sphyraenidae) from the Gulf of Suez, Egypt. Egyptian Journal of Aquatic Research. 2017; 43(1):75-81.
29. Udoh JP, Ukpatu JE, Otoh AJ. Spatial variation in physico-chemical parameters of Eastern Obolo estuary, Niger Delta, Nigeria. J Environ Earth Sci. 2013; 3(12):163-72.
30. King RP. Length-weight relationships of Nigerian freshwater fishes. Naga. 1996; 19(3):49-52.
31. Sparre P. Introduction to tropical fish stock assessment. Part 1. Manual. FAO Fish. Tech. Paper. 1998; 306:1-407.
32. Chigeru, K., and Amachree, D. 2019. Composition, length-weight relationship and condition factor of schilbeidae (siluiri formes) from Agbura landing site, Bayelsa state, Nigeria. GSJ, 7(2).
This article licensed under the Creative Commons Attribution 4.0 International License CC-BY 4.0., which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are properly credited.
