NL Journal of Agriculture and Biotechnology
(ISSN: 3048-9679)
Fungal Biodiversity in Gray Forest Soils of Natural and Agricultural Ecosystems
Author(s) : Irina Senicovscaia, Andriana Danilov, Iurii Rozloga. DOI : 10.71168/NAB.02.03.115
Abstract
The diversity of microscopic fungi in the gray soils of forest and agricultural ecosystems in the central and northern zones of the Republic of Moldova was analysed. The fungal complex of natural gray soils is characterized by high biodiversity and includes by 2 divisions, 8 families, and 14 genera. Arable soils also contain 2 orders and 8 families, but only 12 genera. Ascomycota dominates the fungal composition, accounting for 87.1–100.0% in natural soils and 82.3-100.0% in arable soils. In gray soils under the forest, the predominant genera are Penicillium, Talaromyces and Trichoderma. In arable soils, the genera Aspergillus, Penicillium, Trichoderma, Cladosporium, Verticillium, and Fusarium are present. The greatest declines in abundance and biodiversity were recorded in the arable molic gray soil, caused by a decrease in humus content and a shift in pH toward the neutral zone. Phytotoxic species of the genera Fusarium and Verticillium either appear for the first time or increase their share in fungal biodiversity in arable soils, reaching 6.4–10.0%. The soils did not exhibit any inhibitory effect on the seeds of the test plant. Keywords: Fungi, biodiversity, gray forest soil, phytotoxicity, natural and agricultural ecosystem.
Introduction
Soil fungi are an important and indispensable component of the soil microbial community [8,17]. They have essential functions in carbon and nutrient transformation, formation of the water-stable structure, production of bioactive substances, etc. [30]. Soil fungi make up approximately 30% of the microbial population in the soil rhizosphere. In soil, although there are fewer individual fungi than bacteria, their biomass appears dominant due to their size, constituting the majority of the total biomass of microorganisms [4,11,24,29].
In forest ecosystems, microscopic fungi are an integral heterotrophic component; participating in the degradation of organic matter, the formation of humus and the maintenance of the nutrient cycle [5]. Many studies indicate that fungal structure indicators contain high information content for biomonitoring both natural and anthropogenic ecosystems [4,23]. At the same time, microscopic soil fungi are more likely than other microorganisms to produce phytotoxic compounds [10]. Many species of toxin-forming fungi, commonly found in soil ecosystems, belong to genera such as Aspergillus, Penicillium, Fusarium, Trichoderma, Botrytis, Verticillium [1,9,25].
Fungi are known to grow primarily in low pH or moderately acidic soils, particularly in environments where the soil remains undisturbed [14]. As noted by Sylvia et al. [24], “fungi prefer to dominate soils that are highly stable forms with organic residues having high carbon to nitrogen (C/N) values and slower nutrient recycling times”. It is assumed that gray forest soils in Moldova provide suitable conditions for the investigation of fungal diversity. As we have previously established [19], gray soils cover an area of 284.040 hectares, representing 9.15% of the total soil area in the Republic of Moldova.
They are formed under forests dominated oak (Quercus petraea, Quercus robur), hornbeam (Carpinus betulus), sharp-leaved maple (Acer platanoides), lime (Tilia platyphyllos), ash (Fraxinus excelsior), bird cherry (Prunus padus), and other deciduous species [28]. Their formation is influenced by the prevailing altitudes (140-350 m), climatic conditions (precipitation exceeding 500 mm per year), and the age of geological deposits. These soils are widespread on the Northern Plateau, the Pre-Nistru River hills of Kodru and Tigeche, and are also found fragmentarily in the forest-steppe zone. A characteristic of these soils is the profile differentiation based on the degree of eluviation and illuviation processes [18,27,28]. The peculiarities of the genesis, classification, and geographical distribution of gray soils formed under forest vegetation are discussed in numerous publications [3,6,7,12,18,27].
In this context, assessing the quality status of fungi in gray forest soils across contrasting ecosystems is a highly relevant issue. Such research aligns with soil testing objectives aimed at improving monitoring systems, ecological certification, and biodiversity conservation.
Materials and Methods
Sites: Our research was conducted in the central and northern zones of the Republic of Moldova. Descriptions of the study areas were given earlier [20], but we present them briefly for a better characterization of the objects. The studies were conducted on molic, albic and typical gray forest soils (classification of soils by Ursu) [26,27].
The site with molic gray forest soil (profile 3 under forest; profile 4 under arable) is located near the Grozeshti village in the Nisporeni region. According to pedogeographic zoning, this site is located in the Central Plateau of Kodru Forests, within the region V of Kodru’ Plateau, specifically in the District No.8, characterized by brown and gray forest soils and leached chernozems.
The site with albic gray forest soil (profile 7 under forest; profile 8 under arable) is located near Terebna village in the Edinets region. This site falls within the hilly forest-steppe zone of the Northern Plain (I), specifically in the forest-steppe of the Northern Plateau, within District No. 1, characterized by gray forest soils and clay-allu- vial chernozems.
The site with typical gray forest soil (profile 9 under forest; profile 10 under arable) is also located in the hilly forest steppe zone of the Northern Plain (I), specifically in District No. 5 of gray forest soils and argillaceous chernozems near the Raspopeni village, Sholdaneshti region.
The spatial distribution of gray soils on the territory of the Republic of Moldova and the location of soil profiles with coordinates are presented in Figure 1 and Table 1.
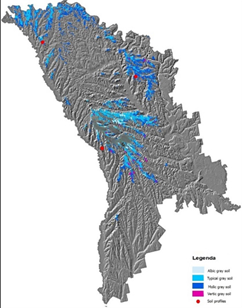
Figure 1: Spatial distribution of gray soils in the country and the location of soil profiles
| Soil | Land use | Profile | WGS 84 coordinate system, decimal degrees | |
| Latitude | Longitude | |||
| Molic gray soil | forest | P3 | 47.01186 | 28.141662 |
| arable | P4 | 47.012184 | 28.141306 | |
| Albic gray soil | forest | P7 | 48.060274 | 27.241933 |
| arable | P8 | 48.060001 | 27.241619 | |
|
Typical gray soil |
forest | P9 | 47.724585 | 28.64117 |
| arable | P10 | 47.72436 | 28.641748 | |
Table 1: Catalog of site coordinates for gray soil profiles
Soils: The studies were conducted on molic, albic and typical gray forest soils (classification of soils by Ursu, 2001; 2022). In the profile 3, the humus content in the Ad horizon (0–17 cm) is 5.44% with pHH2O = 6.70, pHKCl = 5.95, and hydrolytic acidity = 2.92. In profile 4, the humus content in the Ap horizon (0-22 cm) is 3.63% with pHH2O = 7.10, pHKCl = 6.10, and hydrolytic acidity = 2.46.
The humus content in the topsoil of the albic gray soil under forest is 2.93% in the 0–7 cm layer and 2.00% in the 7–30 cm layer. The soil pHH2O in this soil is 6.67 and 5.85, while pHKCl = 5.22 and 4.80; hydrolytic acidity = 3.72 and 9.61 in the aforementioned layers. The arable soil (profile 8) contains 1.77% humus in the 0-32 cm layer. The pHH2O is 6.80, pHKCl is 5.20, and hydrolytic acidity is 3.70.
The humus content in the top layer (0-30 cm) of the typical gray soil under the forest is 3.80%. The pHH2O is 6.25, pHKCl is 5.02, and hydrolytic acidity is 7.84. The humus content in the 0-32 cm layer of the arable soil (profile 10) is estimated at 2.16 %. The pHH2O is 6.55, pHKCl is 4.80, and hydrolytic acidity is 6.05.
Methods: The “natural soil - arable soil” method was used to determine and assess fungal biodiversity. Soil samples for evaluating the microscopic fungal complex and the fungal potential of gray soils in natural forest and agricultural ecosystems were collected from the 0-30 cm layer in accordance with SM ISO 10318-6:2012 [21]. The fungal count was determined by sowing a soil suspension on Chapek’s nutrient medium [13,15,16,31,32]. Soil phytotoxicity was determined using the germinated maize seeds method [22]. The humus content was analysed using the dichromate oxidation method; the actual reaction (pHH2O and pHKCl) was measured by the potentiometric method; hydrolytic acidity was determined by Kappen’s method; and soil moisture was assessed using classical methods [2]. The statistical analysis was performed using MS Excel.
Results and Discussions
Microscopic fungi are widespread in gray soils under forests due to favourable conditions for their activity, in- cluding a regular supply of organic plant residues with a high C/N ratio, an undisturbed soil matrix, and slightly acidic to acidic pH. Research has shown that natural gray soils contain 18.10–75.57 CFU × 103 g-1 of fungi, while arable gray soils contain 14.86–31.99 CFU × 103 g-1 of fungi (Table 2). Fungi in natural gray soils belong to two divisions (Zygomycota and Ascomycota), eight families (Mucoraceae, Trichocomaceae, Hypocreaceae, Davidiel- laceae, Plectosphaerellaceae, Nectriaceae, Chaetomiaceae, and Cordycipitaceae) and 14 genera (Mucor, Rhizopus, Aspergillus, Penicillium, Talaromyces, Trichoderma, Gliocladium, Sepedonium, Acremonium, Cladosporium, Ver- ticillium, Fusarium, Humicola, and Beauveria).
In the fungal community of natural molic gray soil, the dominant position among the identified genera is occu- pied by the family of Trichocomaceae (81.6%). The presence of the families Hypocreaceae (10.9%), Mucorace- ae (2.7%), Chaetomiaceae (2.0%), Plectosphaerellaceae (1.4%), and Nectriaceae (1.4%) was also recorded. The fungal complex in molic gray soil under the forest is dominated by representatives of the genera Penicillium (53.7%), Talaromyces (21.8%), Aspergillus (6.1%), Gliocladium (4.8%), Trichoderma (3.4%), Mucor (2.7%), and Acremonium (2.7%). These fungi account for 95.2% of the total biodiversity of the identified genera (Figure 2).
| Family | Genus | Molic gray soil, forest (P3) | Molic gray soil, arable (P4) | Albic gray soil, forest (P7) | Albic gray soil, arable (P8) | Typical gray soil, forest (P9) | Typical gray soil, arable (P10) |
| Zygomycota | |||||||
| Mucoraceae | Mucor | 1.85 | 0 | 0 | 1.19 | 1.43 | 0 |
| Rhizopus | 0 | 4.63 | 0 | 0 | 0.48 | 0 | |
| Ascomycota | |||||||
|
Trichocomaceae |
Aspergillus | 4.17 | 3.37 | 0.41 | 2.77 | 0 | 0.38 |
| Penicillium | 36.62 | 5.47 | 2.43 | 10.28 | 4.76 | 3.81 | |
| Talaromyces | 14.84 | 0 | 2.83 | 0.79 | 2.86 | 0 | |
| Saccotheciaceae | Aureobasidium | 0 | 0 | 0 | 0 | 0 | 0.38 |
|
Hypocreaceae |
Trichoderma | 2.32 | 6.74 | 5.26 | 2.77 | 1.43 | 2.67 |
| Gliocladium | 3.25 | 0 | 0.81 | 1.19 | 0 | 0 | |
| Sepedonium | 0 | 0 | 1.21 | 0 | 0 | 0 | |
| Acremonium | 1.85 | 0 | 0.40 | 0 | 0 | 0 | |
| Davidiellaceae | Cladosporium | 0 | 2.53 | 6.07 | 1.98 | 3.33 | 1.52 |
| Plectosphaerellaceae | Verticillium | 0.93 | 1.68 | 2.02 | 1.19 | 0 | 1.14 |
| Nectriaceae | Fusarium | 0.93 | 1.68 | 0 | 3.56 | 0 | 0.76 |
| Pleosporaceae | Alternaria | 0 | 0 | 0 | 0 | 0 | 0.76 |
| Chaetomiaceae | Humicola | 1.39 | 0 | 0 | 0 | 0 | 0 |
| Cordycipitaceae | Beauveria | 0 | 0 | 0 | 0 | 0.48 | 0 |
| Unidentified species | 7.42 | 5.89 | 7.28 | 4.75 | 3.33 | 3.44 | |
| Total | 75.57 | 31.99 | 28.72 | 30.47 | 18.10 | 14.86 | |
| LSD0.5 | 6.61 | 1.77 | 1.72 | 1.91 | 1.13 | 0.92 | |
Table 2: Fungal diversity (CFU × 103 g-1 soil) in gray soils of natural and agricultural ecosystems (0–30 cm)
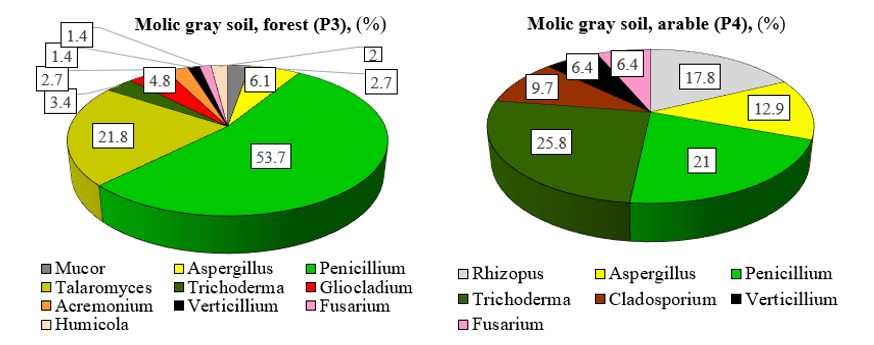
Figure 2: Genus composition of fungi in the molic gray soil of natural and agricultural ecosystem (among the identified genera)
In albic gray soil under the forest, fungal diversity is distributed among the families Hypocreaceae (35.8%), Davidiellaceae (28.3 %), Trichocomaceae (26.5%), and Plectosphaerellaceae (9.4%). The dominant fungal genera in natural albic gray soil are Cladosporium (28.3%), Trichoderma (24.5%), Talaromyces (13.2%), Penicillium (11.3%), Verticillium (9.4%), and Sepedonium (5.7%) (Figure 3). Overall, these genera account for 92.4% of the total identified genera.
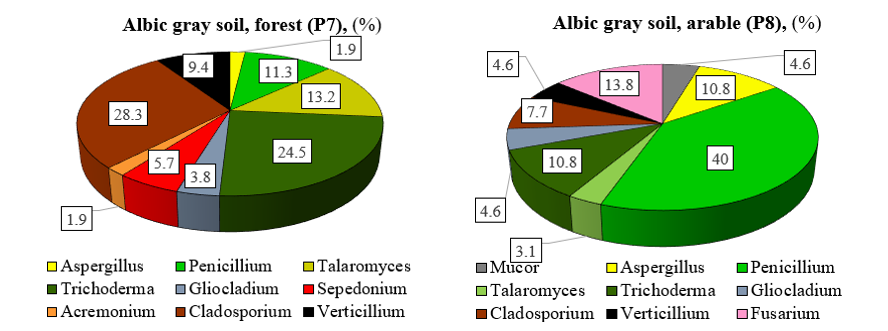
Figure 3: Genus composition of fungi in the albic gray forest soil of natural and agricultural ecosystem (among the identified genera)
The family Trichocomaceae (51.6%) constitutes a major part of the fungal community structure in typical gray forest soil. The proportions of the families Davidiellaceae, Mucoraceae, and Hypocreaceae are 22.6%, 12.9%, and 9.7% respectively. The dominant fungal genera in typical gray soil of natural ecosystems are Penicillium (32.2%), Cladosporium (22.6%), Talaromyces (19.4%), Mucor (9.7%), Trichoderma (9.7%), collectively accounting for 93.6% of the total (Figure 4). The most common and consistent genera of microscopic fungi characteristic of the investigated gray soils in forest ecosystems are Penicillium, Talaromyces, and Trichoderma.
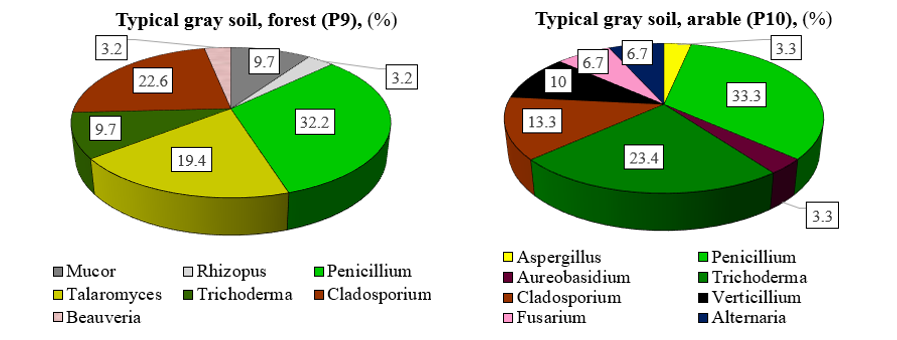
Figure 4: Genus composition of fungi in the typical gray forest soil of natural and agricultural ecosystem (among the identified genera)
The fungal communities in agricultural ecosystems differ significantly from those of natural ecosystems. The total number of fungi in the arable molic gray soil is significantly lower – by 2.4 times less – compared to natural soil. The abundance of fungal communities in arable typical soil is 21.8% lower compared to soil under forest. The numbers of microscopic fungi in natural and arable luvic gray soil are approximately the same (Table 2).
Fungi in arable gray soils belong to two divisions (Zygomycota and Ascomycota), eight families (Mucoraceae, Trichocomaceae, Saccotheciaceae, Hypocreaceae, Davidiellaceae, Plectosphaerellaceae, Nectriaceae, and Pleosporaceae), and 12 genera (Mucor, Rhizopus, Aspergillus, Penicillium, Talaromyces, Aureobasidium, Trichoderma, Gliocladium, Cladosporium, Verticillium, Fusarium, and Alternaria).
In the fungal community of the arable molic gray soil the dominant families are Trichocomaceae (33.9%), Hypocreaceae (25.8%), and Mucoraceae (17.7%). The presence families Davidiellaceae (9.7%), Plectosphaerellaceae (6.5%), and Nectriaceae (6.4%) was also observed. It has been established that the dominant genera in arable molic gray soil are Trichoderma (25.8%), Penicillium (21.0%), Rhizopus (17.8%), Aspergillus (12.9%), Cladosporium (9.7%), and Fusarium (6.4%). Together, these fungi account for 93.6% of the total biodiversity.
Trichocomaceae (53.8%), Hypocreaceae (15.4%), and Nectriaceae (13.9%) were the predominant families in the fungal community of arable albic gray soil. Davidiellaceae (7.7%), Mucoraceae (4.6%), and Plectosphaerellaceae (4.6%) were present in lower abundance. Representatives of genera Penicillium (40.0%), Fusarium (13.8%), Aspergillus (10.8%), Trichoderma (10.8%), and Cladosporium (7.7%) dominate in the fungal complex of arable albic gray soil, comprising 83.1% of the total.
At the family level, fungal diversity in arable typical gray soil is represented by Trichocomaceae (36.7%), Hypocreaceae (23.4%), Davidiellaceae (13.3%), Pleosporaceae (10.0%), Saccotheciaceae (3.3%), Plectosphaerellaceae (6.6%), and Nectriaceae (6.7%). The dominant genera among fungi in arable typical gray soils are Penicillium (33.3%), Trichoderma (23.4%), Cladosporium (13.3%), Verticillium (10.0%), Fusarium (6.7%), and Alternaria (6.7%). Overall, these genera contribute 93.4% to the total fungal community.
It should be noted that phytotoxic species of the genera Fusarium and Verticillium appear for the first time or their number increases in arable gray soils.
The exception is the number of fungi from the genus Verticillium in albic gray soil. Six genera — Aspergillus, Penicillium, Trichoderma, Cladosporium, Verticillium, and Fusarium — are the common microscopic fungi characteristic of all studied soils in agricultural ecosystems.
The assessment of soil fungi biodiversity at the species level within the genus Trichoderma has demonstrated a redistribution in their composition as a result of the arable use of gray forest soils (Table 3). Molic gray soil under the forest contains the species of Trichoderma citrinoviride and Trichoderma lignorum. Natural albic gray soil is caharacterised by the species of Trichoderma lignorum and Trichoderma viride. The species Trichoderma asperellum and Trichoderma harzianum inhabit of the typical gray soil in forest ecosystems.
Arable gray forest soils also contain two Trichoderma species in each subtype: molic gray soil — Trichoderma glaucum and Trichoderma lignorum; albic gray soil — Trichoderma harzianum and Trichoderma lignorum; typical gray soil — Trichoderma citrinoviride and Trichoderma glaucum.
It should be noted that Trichoderma lignorum is the most widespread in forest soils. It is abundant in molic gray soil and in albic gray soil in both forest and agricultural ecosystems.
Along with changes in the state of fungi in arable soils, there is a decrease in humus content and a shift in pH from slightly acidic and acidic to neutral zone. Thus, the humus content in the A horizon of molic gray soil decreases from 3.22–5.44% under forest to 3.30–3.63% on arable land; in albic gray soil, it declines from 2.20–2.93% (Ao and A1 horizons) to 1.77%; and in typical gray soil, from 3.80% to 2.16%, respectively. The natural soil (profile P3) has a slightly acidic reaction in the first two horizons (pHH2O = 6.10–6.70). The arable soil (profile 4) has a neutral reaction in the first three horizons (pHH2O = 7.10–7.20). A similar shift toward the neutral zone is also observed in the other two soils.
| Species | Molic gray soil, forest (P3) | Molic gray soil, arable (P4) | Albic gray soil, forest (P7) | Albic gray soil, arable (P8) | Typical gray soil, forest (P9) | Typical gray soil, arable (P10) |
| Trichoderma asperellum | 0 | 0 | 0 | 0 | 0.48 | 0 |
| Trichoderma citrinoviride | 0.46 | 0 | 0 | 0 | 0 | 1.14 |
| Trichoderma glaucum | 0 | 2.95 | 0 | 0 | 0 | 1.53 |
| Trichoderma harzianum | 0 | 0 | 0 | 1.19 | 0.95 | 0 |
| Trichoderma lignorum | 1.86 | 3.79 | 2.83 | 1.58 | 0 | 0 |
| Trichoderma viride | 0 | 0 | 2.43 | 0 | 0 | 0 |
| Total | 2.32 | 6.74 | 5.26 | 2.77 | 1.43 | 2.67 |
| LSD0.5 | 0.74 | 1.25 | 1.14 | 0.76 | 0.53 | 0.63 |
Table 3: Species composition of Trichoderma fungi under different land management conditions (CFU × 103 g-1 soil, mean values, 0–30 cm)
Phytotoxic biotesting of the investigated soils demonstrated the absence of an inhibitory effect on plants (Table 4). On the contrary, the soils stimulate the growth and development of the test object — maize seeds by the 5th day.
|
Variant (control, soil subtype) |
Mass of germinated seeds (50 seeds), g |
Plantlets length (n=50) | Roots length (n=50) | ||
| cm | % from the control | cm | % from the control | ||
| Control (water) | 14.0 | 3.4 | 100 | 6.4 | 100 |
| Molic gray soil (forest) | 14.0 | 8.1 | 238 | 8.5 | 133 |
| Molic gray soil (arable) | 12.0 | 5.5 | 162 | 8.0 | 125 |
| LSD0.5 | 0.8 | 0.8 | |||
| Albic gray soil (forest) | 13.0 | 6.9 | 203 | 10.1 | 158 |
| Albic gray soil (arable) | 15.0 | 3.8 | 112 | 5.9 | 92 |
| LSD0.5 | 0.6 | 0.8 | |||
| Typical gray soil (forest) | 17.0 | 7.1 | 209 | 7.9 | 123 |
| Typical gray soil (arable) | 12.0 | 5.3 | 156 | 7.4 | 116 |
| LSD0.5 | 0.7 | 0.7 | |||
Table 4: Biometric indices of maize during seed germination in gray forest soils under natural and agricultural ecosystems
The highest stimulating effect was observed in natural soils. Germinated maize seeds had larger plantlets (by 47.3–81.6%) and longer roots (by 6.3–71.2%) in natural gray soils compared to arable gray soils.
Thus, changes in the abundance and diversity of soil fungi, along with an increase in phytotoxic species from the genera Fusarium and Verticillium due to the long-term agricultural use of soils, did not lead to the manifestation of phytotoxic properties in arable gray forest soils.
Conclusions
Microscopic fungi play a crucial role in the biogeocenotic functions of gray forest soils and in shaping their quality. Fungi in natural gray soils belong to two divisions, eight families, and 14 genera, while those in arable gray soils belong to two divisions, eight families, and 12 genera. In natural gray soils, the division Ascomycota constitutes 87.1–100.0% of soil fungi, while in arable gray soils, it accounts for 82.3–100.0%, dominating the fungal composition in both cases. In natural molic gray forest soils, the predominant fungal family is Trichocomaceae (81.6%). In natural albic soils, the dominant families are Hypocreaceae (35.8%), Davidiellaceae (28.3%), and Trichocomaceae (26.5%). In typical forest soils under forest, Trichocomaceae (51.6%), Davidiellaceae (22.6%), and Mucoraceae (12.9%). The genera Penicillium (11.3-53.7%), Talaromyces (13.2–21.8%) and Trichoderma (3.4–24.5%) are common in gray soils under forest.
Long-term agricultural use of forest soils leads to the degradation of fungal community, manifested by a decline in fungal abundance, a shift in diversity, or both processes occurring simultaneously.
The redistribution of species from the genus Trichoderma was observed as a result of the arable use of gray forest soils. The greatest declines in abundance and biodiversity were recorded in arable molic gray soil, where fungal abundance decreased by 2.6 times, and the number of genera declined from 10 to 7. We note that the share of families Trichocomaceae, Hypocreaceae, and Davidiellaceae in the fungal diversity of arable gray forest soils decreases from 83.9–92.5% to 69.4–76.9% as a result of long-term agricultural use. The genera Aspergillus (3.3–12.9%), Penicillium (21.0–40.0%), Trichoderma (10.8–25.8%), Cladosporium (7.7–13.3%), Verticillium (4.6–10.0%), and Fusarium (6.4–13.8%) are present in all arable soils, collectively accounting for 82.2–90.0%. Phytotoxic species from the genera Fusarium and Verticillium either appear for the first time or their share in fungal biodiversity increases to 6.4–10.0% in arable soils.
Changes in the abundance and diversity of fungi in arable soils are caused by a decrease in humus content and a shift in pH from slightly acidic and acidic to neutral due to the mixing of the upper soil layers.
Phytotoxic biotesting of the investigated soils has demonstrated no inhibitory effect on plants. The maximum stimulative effect was observed in soils of natural ecosystems.
References
1. Abbas, H. K., Mirocha, C.J., Kommedahl, T., Vesonder, R.F., & Golinski, P. (1989). Production of trichothecene and non trichothecene mycotoxins by Fusarium species isolated from maize in Minnesota. Mycopathologia, 108, 55-58.
2. Arinushkina, E.V. (1970). Guideline for chemical analysis of soils. MSU Publishing. (in Russ).
3. Baltianski, D. M. (1979). Soils of the Central Kodru. Chisinau: Stiinta. (in Russ).
4. Evdokimova, G. A. (2014). Soil microbiota as a factor of soil resistance to pollution. Theoretical and Applied Ecology, 2, 17 24. http://envjournal.ru/ari/v2014/v2/files/14202.pdf
5. Gadd, G. M. (2007). Geomycology: biogeochemical transformations of rocks, minerals, metals and radionuclides by fungi, bioweathering and bioremediation. Mycologia, 111, 1, 3-49.
6. Grati, V. P. (1975). Nature of textural differentiation of forest soil profile in Moldova. Pochovedenie, 8, 15-19. (in Russ).
7. Grati, V.P. (1977). Forest soil of Moldavia and their rational utilization. Chisinău: Stiinta. (in Russ).
8. He, L., Rodrigues, J. L. M., Soudzilovskaia, N. A., Barcelo, M., Olsson, P. A., Song, C., et al. (2020). Global biogeography of fungal and bacterial biomass carbon in topsoil. Soil Biology and Biochemistry, 151, 108024.
9. Ismaiel, A.A., & Panenbrock, J. (2014). The effects of patulin from Penicillium vulpinum on seedling growth, root tip ultrastructure and glutathione content of maize. Eur. J. Plant Path., 139, 497-509.
10. Ismaiel, A.A., Papenbrock, J., & Jutta. (2015). Mycotoxins: Producing Fungi and Mechanism of Phytotoxicity. Agriculture, 5(3), 492-537. https://doi.org/10.3390/agriculture5030492
11. Korneikova, M. V., & Nikitin, D. A. (2023). Soil microbiome in the zone of impact of emissions from the mining and steel plant Pechenga nickel (Murmansk region). Pochvovedenie, 5, 676-688. (in Russ).
12. Krupennikov, I. A., & Podymov B. P. (1987). Classification and systematic list of soils of Moldavia. Chisinau: Stiinta. (in Russ).
13. Lacatusu, R., N. Rizea, D. Stefănescu, V. Tănase, N. Vrînceanu, M. Preda, A. Lăcătusi, M. Matei, & S. Matei. (2011). Metode de analiză chimică si microbiologica (utilizate în sistemul de monitorizare a solurilor). M. Dumitru & A. Manea (Coord).EDITURA SITECH, Craiova, România. (in Rom).
14. Lavelle, P., & Spain, A. V. (2005). Chapter 3: Soil Organisms. Soil Ecology. Springer: New Delhi.
15. Litvinov, M. A. (2013). Determinator of microscopic soil fungi. Ripoll Classic. (in Russ).
16. Methods of soil microbiology and biochemistry (1991). Zvyagintsev, D. G. (Ed.). MSU Publishing. (in Russ).
17. Mirchink, T. G. (1988). Soil mycology: Manual. MSU Publishing. (in Russ).
18. Podymov, B.P & Krupenikov, I.A. (1984). Classification of soils. Gray forest soils. Soils of Moldavia Chisinau: I.E.P. Stiinta, 42-43. (in Russ).
19. Rozloga, Iu. (2015). Evaluarea stării ecologice a resurselor de sol cu utilizarea sistemului geoinformational. Conferint aInternatională ‘‘Mediul si schimbarea climei: de la viziunea la actiune”, Chisinău: S.n., Tipografia ‘‘Simbol-NP”, 161-164.(in Rom).
20. Senicovscaia, I., Danilov A. & Danilov A. (2021). Biodiversity of edaphic fauna in gray forest soils of the Republic of Moldova. Current Trends in Natural Sciences, Publisher: University of Pitesti, EUP. 10 (19), 134-141. https://doi.org/10.47068/ctns.2021.v10i19.018
21. SM ISO 10318-6:2012. Soil quality – Sampling. Part 6: Guidance on the collection, handling and storage of soil under aerobic conditions for the assessment of microbiological processes, biomass and diversity in the laboratory.
22. SM SR ISO 11269-1:2012. Soil quality – Determination of the effects of pollutants on soil flora. Part 1: Method for the measurement of inhibition of root growth.
23. Svistova, I.D., & Nazarenko, N.N. (2022). Ecological trend of mycobiome succession in chernozem of an old botanical garden. Theoretical and Applied Ecology, 3, 142-148. http://envjournal.ru/upload/449_22318.pdf
24. Sylvia, D. M., Fuhrmann, J. J., Hartel, P. G., & Zuberer, D. A. (2005). Principles and Applications of Soil Microbiology (Pearson Prentice Hall, UPPER Saddle River, New Jersey).
25. Tseng. T. (1993). Mycotoxins produced by Fusarium spp. of Taiwan. Bot. Bull. Acad. Sin., 34, 261-269.
26. Ursu, A. (2001). Clasificarea solurilor Republicii Moldova (Editia II). Chisinău, SNMSS. https://ru.scribd.com/ document/321198850/03-Clasificarea-Solurilor-Moldova (in Rom).
27. Ursu, A. (2011). Solurile Moldovei.: Ch.: I.E.P. Stiinta. http://editurastiinta.md/solurile-moldovei (in Rom).
28. Ursu, A., Overcenco, A., Curcubăt S., & Miron A. (2023). Solurile pădurilor din Republica Moldova. Chisinău: S.n. https://infoinvent.md/assets/files/inf/2.C.23.pdf (in Rom).
29. Wallander, H., Nilsson, L.O., Hagerberg, D., & Bååth, E. (2001). Estimation of the biomass and seasonal growth of external mycelium of ectomycorrhizal fungi in the field. New Phytologist, 151 (3), 753-760. https://pubmed.ncbi.nlm.
nih.gov/33853251/
30. Xu, X., Thornton, P. E., & Post, W. M. (2013). A global analysis of soil microbial biomass carbon, nitrogen and phosphorus in terrestrial ecosystems. Global Ecology and Biogeography, 22 (6), 737-749. https://doi.org/10.1111/geb.12029
31. Zenova, G.M., & Kurakov, A.V. (1988). Methods of determining the structure of soil actinomycetes and fungi complexes. MSU Publishing. (in Russ).
32. Zvyagintsev, D.G., Babieva, I.P., & G.M. (2005). Soil Biology: Manual for university students. 3rd edition, revised, suppl. MSU Publishing. (in Russ)
This article licensed under the Creative Commons Attribution 4.0 International License CC-BY 4.0., which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are properly credited.
