NL Journal of Agriculture and Biotechnology
(ISSN: 3048-9679)
Effect of Zinc on Germination Behavior and Protein Profile of Helianthus Annuus L. Seedlings
Author(s) : Raveendran M, Anjana M. DOI : 10.71168/NAB.02.01.103
Abstract
Present study was carried out to evaluate the effect of different concentrations of zinc (1, 5, and 10mM) on biomass production and protein profile of Helianthus annuus seedlings after 72 hours of germination. Maximum seedling growth was observed in 1mM ZnSO4 treatment and the germination rate showed a gradual reduction in higher concentrations. The inhibitory effect of Zn toxicity on root and hypocotyls growth was proportionate to the concentrations of Zn. Electrophoretic analysis of the protein fractions revealed that during germination of seedlings growing up to 72 hrs., the intensities of bands slightly increased in 10mM concentration. Results strongly indicate that in spite of significant morphological changes, the protein distribution pattern of Helianthus annuus remains unaltered due to Zn toxicity and 10 mM concentration imposes no toxicity in the metabolism of seedlings. Keywords: Biomass, Helianthus annuus L., Growth, Electrophoretic analysis
Introduction
Zinc is an essential micronutrient for plants, exists Zn2+ which plays a key role as a structural constituent or regulatory co-factor of a wide range of different enzymes and proteins in many important biochemical pathways and these are mainly concerned with carbohydrate metabolism, both in photosynthesis and in the conversion of sugars to starch, protein metabolism, auxin (growth regulator) synthesis, pollen formation, the maintenance of the integrity of biological membranes, the resistance to infection by certain pathogens [1]. Even though a small amount of Zn is required for plants (5–100 mg/kg) enough of this element is not available, the plants suffer physiological stress resulting from the incompetence of several enzyme systems and other metabolic actions interrelated to Zn [2]. During the process of seed germination, zinc absorbed from the soil mostly get retain in the root, but a portion is translocated to the shoots. Sunflower (Helianthus annuus L.) plant is one of the most edible seed crops growing in harsh climatic regions. It also has good water use efficiency. The seeds contain high oil content (35%-48%), Helianthus annuus L. has a high level of lipid (69%) easily explains the importance of the sunflower plant. Helianthus annuus is a rich source of healthier edible oil [3,4,5]. According to [6] H. annuus contains 40-47% oil, 20-27% protein.
Helianthus annuus contains about 69% lipid and hence the plant is economically important and protein of H. annuus consists of albumin, globulin, prolamin and glutelins [7]. Oil seed crops play a vital role in broiler food [8]. High temperature and drought during the flowering and seed filling stage decrease the yield in sunflower. Even though Zn plays an important role as a mineral nutrient, beneficial roles of the metal have been studied in many plants [9,10]. Effect of zinc on germination pattern and protein content distribution has not yet been studied in detail.
Materials and Methods
Collection of seeds
Mature seeds of Helianthus were purchased from the local market and the healthy seeds were selected, then surface sterilized by immersing in 5% (v/v) sodium hypochlorite for 10 minutes and rinsed three times in sterile water, sun dried and used for the experiments.
Standardized concentrations for optimal growth and inhibitory effect
Concentrations such as 1mM, 5 mM, and 10 mM weighed ZnSO4 salt were dissolved in glass distilled water and were used for the experiment. Glass distilled water was used as the control.
Experiment layout (Petridis culture)
The seeds collected as described above were thoroughly washed with glass distilled water. In order to break the seed dormancy, trial experiments were conducted with distilled water for getting uniform and cent percent germination. Uniformly sprouted ten seeds were placed in each of 9 cm sterilized Petri dishes lined with Whatman No.1 filter paper and 5 ml of each solution (1 mM, 5 mM, and 10 mM ZnSO4) and glass distilled water (control) were added to appropriate petri dishes containing seeds and kept under dark for germination at 25 ± 2°C. After sprouting the seeds were exposed to continuous light (45µEm2s - 1) at 25 ± 2°C. A treatment solution in each Petri dish was changed after every 24 hrs. and fresh solution (5 ml each) was added. Each experiment was replicated a minimum of five times.
Germination Percentage
To determine the germinability of seeds, a sample consisting of 50 seeds were soaked in water. After 5-6 hrs. excess water was decanted and the seeds were kept for germination in Petri dish at room temperature. The number of sprouted seeds were counted, and germination percentage was calculated for a period of 72 hours. The seeds were germinated with the emergence of the radical. The rate of germination percentage was estimated by using a modified Timsons’ index of germination velocity according to [11] as stated below:
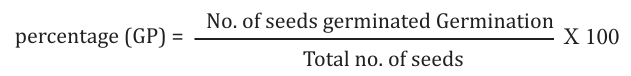
Dry weight determination
Samples were taken in a pre-weighed container and after taking fresh weight; these containers were kept in an oven at 100ºC for 1 hour and until at 60ºC constant weight was obtained.
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
Preparation of protein sample: For the preparation of crude protein extracts of Helianthus annuus, 200 mg each form control, 1 mM, 5 mM and 10 mM of Helianthus composite (root, hypocotyl, and cotyledon), kept at -20°C were weighed and homogenized in a chilled glass mortar and pestle with 400 µl of 0.2 M Tris-HCl buffer (pH 8). The homogenates were centrifuged at 3000rpm for 10 minutes. For analysis by SDS-PAGE, 25 µl supernatant from control and each concentration, and 10 µ protein markers were pipetted out into individual vials. To each of these vials 15 µl of sample loading buffer (tris buffer of pH 6.8, SDS, β Mercaptoethanol which reduces disulfide bonds, sucrose/glycerol to increase the density and bromophenol blue as tracking dye) was added and the vials were heated in boiling water bath for 5 minutes to denature the protein sample.
Electrophoresis: Marker and the prepared protein samples were added to electrophoretic wells and the order in which the samples were loaded is noted. Set the voltage at 100V and switch on the power supply until the dye front reaches 0.5 cm above the bottom of the gel. Resolution of the protein bands is gently increased by applying the samples onto a short stacking gel on top of the separating gel. Differences in pH and composition between these two gels cause the sample to be concentrated into narrow bands by isotachophoresis. As the samples migrate through the separating gel, proteins get resolved depending on their molecular weight. Electrophoresis stops when the dye front reaches the bottom of the gel.
Visualization of proteins: The gel after electrophoretic run was separated and washed with water by transferring into a tray. Then the gel was inserted into staining solution (20 ml Ezee blue) for overnight, and finally the gel was inserted into de-staining solution until the bands appeared and image analysis was performed [12]. The tray was placed on a rocker intermittently every 10 to 15 minutes for uniform staining and washing. Generally, proteins are colorless and hence cannot be visualized directly. So suitable dyes are used to visualize them. Identification of protein bands was done by comparing with similar process marker protein along with the samples
Statistical analysis
All the experiments/treatments were repeated minimum for five times and mean values were calculated and mean values were taken. Standard Error was calculated from mean values and (SE) standard deviation (SD).
Results and Discussion
Seed germination
Pattern of seed germination percentage due to Zn treatment is given in (Fig: 1). The germination percentage of Helianthus annuus seeds in control showed only 95%. Cellular events and metabolic changes of seed reserves undergo drastic changes during imbibition followed by germination and seedling development. The reduction of germination percentage indicates the determination of the quality of seeds, since the seeds were purchased from open market and cent percent germination rate cannot be expected mainly due to the lack of proper drying storage as per [13]. Mobilization of stored reserves of seed is a major event in seedling development occurring after seed germination. This process requires the participation of many enzymes of which a substantial number are synthesized de novo and others are activated under optimum condition of temperature and water relations. Cell division is a prerequisite and cell elongation is essential for emergence of radicle and plumule leading seedling growth. Zinc treatment resulted in a gradual reduction of germination in the concentration of 1 mM, while 5 mM concentration showed a more inhibitory effect in the rate of germination. A prominent inhibitory effect was noticed with increasing concentration of Zn, i.e., 10 mM in which only 37% of seeds were germinated. Followed by inhibition, respiratory enzymes are activated consequently activation of glycolytic pathway also become active. As a prerequisite for germination processes, the development of polysome and synthesis of mRNA results in qualitative and quantitative increase in protein synthesis [7]. The inhibitory effect of ZnSO4 has been reported in tomato seeds when the concentration was 5 mM [14,15].
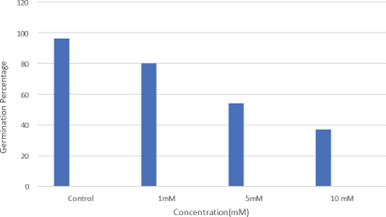 Fig1: Effect of Zinc on seed germination of Helianthus annuus
Fig1: Effect of Zinc on seed germination of Helianthus annuus
Seedling morphology
Zinc stresses the enzyme such as amylase, protease and lipase under stress impact. These enzymes play a crucial role in breaking time of starch, protein and lipid to provide energy for seedling growth and serve the impact of resultant stresses on enzyme activity can influence germination and overall seedling development. As the Zn concentration increased the degree of sprouting of the seeds decreased and towards the higher concentration (5 mM), the colour of the cotyledon was not green as that of control. Zinc form complexes with enzymes and disrupts their normal function altering their conformation and interfering with substrate binding [16]. Root in control and low concentrations were slightly brownish (fig. 6) in colour and showed root hair development. While at higher concentration (10 mM) root hair development was not observed (fig. 6). In higher concentration (5 mM) the length of root was inhibited. Root tips showed a pale black colour (fig. 6) and did not develop further and hypocotyls showed bending nature. Catabolism of lipids of H. annuus seed germination involves the activity of many enzymes and water. In 10 mM Root shows reduced growth than hypocotyls and root tips were black and cotyledons are partially open and green in colour. Hypocotyl growth is more inhibited than root. The hypocotyl is coily and stunted in nature and green in colour. Zinc stress disrupts the balance of essential metal within the seed.
Seedling growth
Changes in root and hypocotyl length of Zn treated seedlings of H. annuus (Fig. 2) showed variations in comparison with the control. Maximum seedling growth was noticed in the seeds treated with 1 mM ZnSO4. It showed an increase in root and hypocotyl length than the control. Poor germination and suboptimal seedling growth constitutes as prime function is lowering yield and seed oil content in sunflower (H. annuus). Least seedling growth was observed in the seeds treated with higher concentration (10 mM). The length of root and hypocotyl of higher concentration (10 mM) was less than that of control. Zinc treatment enhanced seedling emergence rate and vigor. Zinc improved root length along with their fresh weight and dry weight [17]. According to these authors, zinc is a superior priming agent at 5 mM. It resulted in a higher root, shoot length along with their fresh and dry weight. Significant variation in the protein profile of seed treated with zinc and that of the control is indicative of the metabolism of seed reserve during germination of both control and experimental (Fig.2). A linear decrease of root and hypocotyl length was found in all the treatments of H. annuus treated with Zn. A maximum promotion of growth in root and hypocotyls was found 1 mM ZnSO4 compared to control and maximum inhibitory growth was found in a higher concentration (10 mM) of Zn treated one. It can be interpreted that 1 mM ZnSO4 stimulates seedling growth in terms of root length, hypocotyls and root hair development compared to the control. Catabolism of storage proteins of H. annuus which is in the range of 22-27% [4] occurs during imbibition and get increased during germination.
Whereas higher concentration of 5 mM and 10 mM ZnSO4 solution imposes growth inhibition as opined earlier [14,15]. Growth in terms of dry weight of root and hypocotyl of H. annuus seedlings treated with Zn is given in (Fig. 3). Initially in a lower concentration of Zn (1 mM), there occurred a promotion in dry weight of root than the control. But in higher concentrations (5 mM and 10 mM) the dry weight of root declined. In the case of dry weight of hypocotyl also the maximum weight was observed in control. The dry weight of hypocotyl showed a sharp decline as the concentration increases. Dry weight of cotyledon of H. annuus seedling is shown in (Fig. 4.). In cotyledon, a slight increase in the dry weight was noticed with increasing concentration of Zn treatment. Dry weight of cotyledons in the lower concentration (1 mM) was slightly greater than control. In high concentration the dry weight is greater than control and 1 mM. Many enzymes require Zn2+ for their activity for the biosynthesis of metabolites and chlorophyll pigments [18]. The protein band of seedlings synthesized to zinc treatment exhibits more intensity and bandwidth (Fig. 6) indicates the synthesis of chlorophyll pigments since seedlings become greener in color when the concentration of zinc increased.
In H. annuus germination is epigeal and cotyledon becomes green in color in the seedling stage. During germination and seedling growth, catabolism of seed reserves occurs in seeds in H. annuus. Since the seeds contain carbohydrates, protein and lipids which carbohydrate about 69% of the total seed reserves. Hydrolysis of storage protein in their component amino acid requires a class of enzymes such as endopeptidases, aminopeptidases and carboxypeptidases resulting in the formation of essential components of storage protein. In addition, protease enzymes are synthesized to provide energy for metabolism as well as new enzyme synthesis essential for catabolic reactions of seed reserve mobilization. Zinc is an important constituent of dehydrogenase. [18] in general and glutamate dehydrogenase in particular content is abundant and become active during germination because several dehydrogenases play vital roles during respiration and other energy requiring metabolic changes.
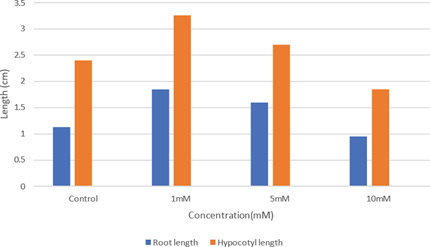
Fig 2: Effect of Zinc on growth of Helianthus annuus seedling parts after 72 hours of germination
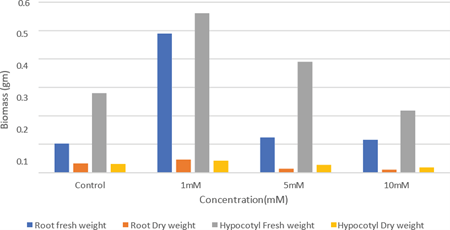
Fig 3: Effect of zinc on Root and Hypocotyl biomass of Helianthus annuus after 72 hours of germination.
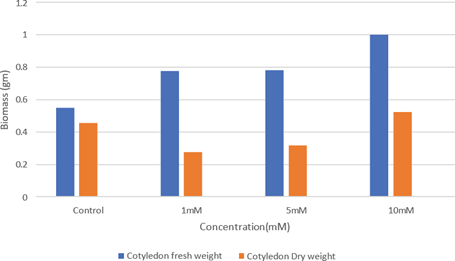
Fig 4: Effect of zinc on Cotyledon biomass of Helianthus annuus after 72hours of germination
The abundant storage reserves of H. annuus seed is lipids, the catabolism of lipid is the main source of energy for germination. Catabolism of lipids, particularly dry acylglycerols which are the major storage lipid in seeds involved different metabolic pathways such as degradation of glycerols and fatty acids. Particularly beta oxidation requires many enzymes in which Zn2+ plays are essential as component as well as a factor for their activity. In the present study PAGE profile shows significant difference the zinc treated seedling compared to the control.
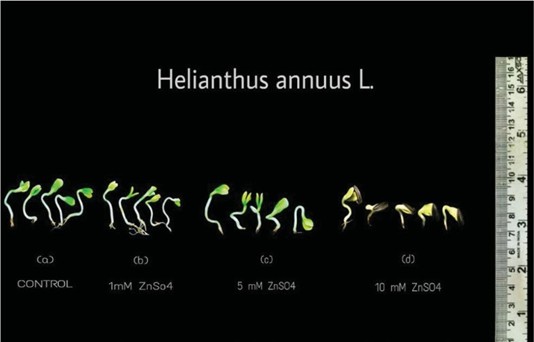
Fig 5: Seed germination pattern of Helianthus annuus treated with ZnSO4 after 72hrs
Analysis of SDS-PAGE
The results pertaining to the effect of zinc on protein content is presented in (figure 6) This technique can be used to separate protein with relative molecular weight not smaller than 10KD protein with low molecular weight shows lower affinity binding to SDS and is not suitable to be separated using basic Leammli SDS-PAGE method [15].
A total of 6 bands ranging from 43 k Da-29 k Da were recognized. In Lane 1 (control) the first 3 bands have higher intensity, but the last 3 bands have low intensity. In lane 2 when compared with control the first 3 bands have low intensity, but the last 3 bands have high intensity ie., when compared with control intensity decreases towards higher concentration in the first 3bandsand intensity increases towards higher concentration in the last 3 bands. In 10 mM the first 3 bands have higher intensity when compared with control, and intensity decreases towards the last 3 bands. Protein bands of PAGE profile of the control and treatment of 1 mM are very feeble compared to the other treatments. Presumably reason for the observation indicates no inhibitory effect rather slight stimulatory confirming other growth parameters described above.
Feeble intensity of protein bands of the control and 1 mM treatment clearly shows the increase in protein content and involvement of increased metabolism of the seedlings in all parts like root, hypocotyls and cotyledons whereas dark and thick protein bands of treatment at 5 mM and 10 mM show reduced protein content and deranged metabolism since Zn induces inhibitory or due to Zn toxicity in H. annuus. In 10 mM concentration first 3 bands showed higher intensity when compared with control, and intensity decrease towards the last 3 bands. Zinc is important and essential in protein metabolism. The amount of protein in Zn deficient plant is dramatically reduced [19]. In the present study PAGE profile shows significant difference the zinc treated seedling compared to the control.
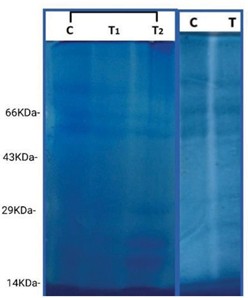
Fig 6: SDS-PAGE Protein profile of H. annuus seedlings treated with control 1 mM, 5 mM, 10 mM ZnSO4 after 72 hrs of germination. Lane 1- Marker, Lane 2- Control, Lane 3- 1 mM concentrations, Lane 4- 5 mM concentrations, and Lane 5- 10 mM concentrations
Conclusion
Effect of zinc toxicity was investigated in seeds of Helianthus annuus by germinating the seeds in different concentrations of ZnSO4. Seeds were treated with 1, 5 and 10 mM ZnSO4 under Petri dish conditions. Germination rate, seedling growth after 72 hours of germination in terms of seedling length and biomass was determined. Germination and seedling growth of H. annuus seeds are positively affected by zinc treatment and zinc can be used as a priming agent for seed germination. Protein profile using SDS-PAGE was another parameter to study the effect of zinc. Results showed a slight stimulatory effect at 1 mM concentration than the control while treatments of 5 mM and 10 mM concentrations are inhibitory to germination and seedling growth. Protein bands of control and 1 mM treatment were feeble compared to the other treatments. The stimulatory effect of Zn at 1mM concentration manifested in germination rate, seedling growth, and protein profile is indicative of the nutrient potential of Zn in mineral nutrition. Effect of Zn at 5 and 10 mM reveals the metal toxicity expressed in stunted growth of seedlings and inhibited protein metabolism.
References
1. Alloway B.J. (2008). Zinc in Soils and Crop Nutrition. International Zinc Association.
2. Baybordi A. (2006). Zinc in Soils and Crop Nutrition. Parivar Press.
3. Chowdhury, A.R., T.K. Parbhakarsetty and T.K. Nagararhna. 2010. Growth and yield of sunflower as influenced by micro nutrients. Karnataka J. Agri. Sci., 23: 495-496.
4. Faisal, M., Iqbal, M. A., Aydemir, S. K., Hamid, A., Rahim, N., El Sabagh, A., Khaliq, A., and Siddiqui, M. H. (2020). Exogenously foliage applied micronutrients efficacious impact on achene yield of sunflower under temperate conditions. Pak. J. Bot.52(4):1215-1221.
5. Khan, S., Choudhary, S., Pandey, A., Khan, M. K., & Thomas, G. (2015). Sunflower oil: Efficient oil source for human consumption. Emergent Life Sciences Research, 1, 1–3.
6. Ahmad, R., and N. Jabeen. 2009. Demonstration of growth improvement in sunflower (Helianthus annuus L.) by the use of organic fertilizers under saline conditions. Pakistan Journal of Botany 41: 1373–1384.
7. Bewley, J. D., Black, M. (1994). Seeds: Physiology of development and germination.2 nd Edition Plenum Press, New York, 445.
8. F.van Assche and H. clijsters. (1990). Effect of metals on enzyme activity in plants. Plant cell and environment 13,195-206.
9. Espen L, Pirovano L, Cocucci S.M. (1997). Effects of Ni2 during the early phases of radish (Raphanus sativus) seed germination. Environ Exp Bot, 38, 187–197.
10. Munzuroglu O., & Geckil H. (2002). Effects of Metals on Seed Germination, Root Elongation, and Coleoptile and Hypocotyl Growth in Triticum aestivum and Cucumis sativus. Archives of Environmental Contamination and Toxicology, 43, 203–213.
11. Khan, M.A. and Ungar, I.A. (1997). Effect of thermoperiod on recovery of seed germination of halophytes from saline conditions. American Journal of Botany, 84, 279-283.
12. Nayer Mohammadkhani, & Reza Heidari. (2008). Effects of Drought Stress on Soluble Proteins in two Maize Varieties. Turkish Journal of Biology, 32.
13. ISTA (1985) International rules for seed testing. Seed Science and Technology 13, 299–513.
14. Taylan Kosesakal, T. and Muammerunal, M. (2012)- Effects of Zinc toxicity on seed germination and plant growth in Tomato (Lycopersicon esculentum mill)., Fresenius environmental Bulletin,21(2),315-324.
15. Metty J Anto and Raveendran M. (2023). Study on the effect of zinc on biomass production and protein profile of Zea mays L. seedlings IJSDR, 8(4) 2076-2082.
16. Bewley, J. D., Black, M. (1985). Seeds: Physiology of development and germination. 1st Edition Plenum Press, New York, 125.
17. Mokhtari, N.E.P., Kızılgeçi, F., Ahmed, R., Iqbal, M.A. (2022). Exploring zinc and boron chemo-priming effects on low-vigour seed germination and seedling establishment of sunflower (Helianthus annuus L.) Turkish Journal of Food and Agriculture Sciences 10:1966-1971.
18. Taiz, L., Zeiger, E., Møller, I. M., & Murphy, A. (2015). Plant physiology and development (6th ed.). Sinauer Assoc Inc., Sunderland.
19. Broadley M.R., White P.J., Hammond J.P., Zelko I & Lux A. (2007). Zinc in plants. New Phytologist, 173, 677 –702.
This article licensed under the Creative Commons Attribution 4.0 International License CC-BY 4.0., which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are properly credited.
