NL Journal of Medical and Pharmaceutical Sciences
(ISSN: 3108-0502)
Visible-Light-Active Kaolinite- TiO₂/rGO Hybrid Photocatalysts for Environmental Cleanup of Persistent Pollutants
Author(s) : Nahida Nargis, Prabal Barua*, Noor Mohammad. DOI : 10.71168/NMP.01.02.108
Abstract
Introduction: The treatment of water which is contaminated with dyes and medicines poses a serious threat to the environment and health. Treatment of wastewater containing these contaminants proves to be difficult using conventional methods. Using titanium dioxide (TiO₂) has been effective in removing these pollutants through photocatalysis. However, fast charge recombination and low band gap prevent practical use. Several studies in recent years have focused on the use of kaolinite, a low-cost and abundant type of clay, to synthesise composite photocatalysts to boost their performance. Methods: This article discusses new developments in photocatalytic systems utilising kaolinite as a material type, with a particular focus on their structural characteristics, synthesis process, and mechanisms. The surface charge features, available hydroxyl groups, and the layered arrangement result in the efficient transfer of charges and the adsorption of pollutants to kaolinite. Additionally, kaolinite composites have been modified to enable activation by visible light. Results: The hybrid materials containing kaolinite exhibit high performance in terms of pollutant degradation, hydrogen evolution, and antibacterial properties compared to single-component semiconductors. Although laboratory-scale results can be promising, several challenges, including large-scale fabrication, gaining detailed insight into the dynamics of interfaces and electrostatics, and synthesizing in an environmentally friendly manner, remain. Conclusion: Further research is necessary to ensure the optimal utilization of available, stationary, and stable kaolinite photocatalysts in the large-scale elimination of contaminants. Among them is the idea that hybrids of kaolinite and TiO₂ reduced graphene oxide (rGO) have high potential in dye treatment, pharmaceutical, and other incompetent water pollutants due to the possibility of excellent adsorption and dispersion of semiconductor nanoparticles that result in optimal charge separation and photocatalytic performance during purification of visions of light. Keywords: Kaolinite-based photocatalysts, Titanium dioxide (TiO₂), Reduced graphene oxide (rGO), Photocatalytic degradation, Water pollution remediation.
Introduction
The contamination of water with synthetic dyes and traces of pharmaceuticals is an aspect that constantly affects and critically impacts the environment and human health. Besides being toxic and bioaccumulative, these pollutants are also very resistant to removal using conventional water treatment methods, including biological degradation, coagulation, and adsorption [1]. To address the shortcomings of TiO₂, including its decreased activity in the visible light spectrum and the high rate of charge carrier recombination, scientists have considered various modification techniques.
One potential solution is to combine TiO₂ with natural clay minerals, such as kaolinite, which can significantly enhance charge separation, light absorption, and photocatalytic productivity [2].
Photocatalysis utilises either sunlight or artificially produced light to trigger semiconductor materials to generate the redox reaction, which enables the mineralisation of a broad spectrum of pollutants without the formation of secondary waste [6]. Among the semiconductor photocatalysts, titanium dioxide (TiO₂) is highly attractive due to its chemical stability, non-toxicity, and high oxidative potential [7]. However, its experimental value is hampered by a large bandgap (approximately 3.2 eV), which makes it efficient only in the ultraviolet spectrum, and an effective recombination of photogenerated electron-hole pairs, both of which limit its response to natural sunlight [3].
The layered structure of kaolinite enhances the adsorption of cationic pollutants by increasing the number of layers, along with its hydroxyl-reactive, negatively charged surface. It improves the uniform dispersion of semiconductor nanoparticles. This increases the number of active sites available, enhances charge transfer, and ultimately leads to higher photocatalytic efficiency. The reaction between kaolinite and TiO₂, mediated by Ti nearest neighbours through either Ti-O-Si or Ti-O-Al bonds, forms a fixed interface. It optimises the separation of charges, and the mirror electron-hole pairs thus generated are not recombined, which constitutes a significant drawback in photosynthesis [4,5].
Recent developments have also incorporated materials such as reduced graphene oxide (rGO) and graphitic carbon nitride (g-C3N4) into kaolinite compositions, creating ternary and quaternary heterojunctions that have been shown to increase photocatalytic activity. The interaction of kaolinite, TiO₂, and rGO or g-C3N4 increases the movement of charge carriers and minimises recombination losses, making these solids more effective in the destruction of pollutants. Ternary kaolinite/ TiO₂/rGO nanosystems have also been shown to exhibit nearly 100% degradation of methylene blue and other dyes under visible light, making them preferable over binary and single-metal systems. Based on the latter, kaolinite/C3N4 and Kaolinite/ZnO composites have demonstrated a better visible light absorption capability and charge separation, thereby broadening the range of kaolinite materials for environmental remediation [6,7,8].
The use of synthesis techniques such as sol-gel, hydrothermal, co-precipitation, and mechanochemical techniques is employed to optimise the dispersion of semiconductors on kaolinite surfaces. These techniques enable the management of particle size, regulation of porosity, and enhancement of the regularity of semiconductor placement, resulting in increased photocatalytic efficiency. To illustrate the point, sol-gel-produced kaolinite/TiO₂ materials tend to exhibit high crystallinity and good particle dispersibility, which translates to increased mobility of charge carriers and enhanced photocatalytic effectiveness. The synthesis conditions (calcination temperature, premolar concentration, and pH) are also adjusted to tune the phase composition, surface chemistry, and stability of the synthesised products [9,10].
Although considerable breakthroughs in the degradation of model pollutants have been reported in laboratory studies, the behaviour of kaolinite-based photocatalysts on complex real-world wastewater, consisting of various organic and inorganic species, remains largely unexplored [11,12]. It is also crucial for the long-term stability, reusability, and environmental Fitness of these materials, as well as the leaching and transpiration of nanoparticles, and the secondary pollution in matters of overall practical execution [13,14].
The principle of charge separation and electron transfer at heterojunctions on kaolinite is not fully understood. The data is crucial in terms of the brightness of photocatalytic systems, their pilotage, and their usage during lab tests and in monitoring water treatment procedures. Such mechanisms require sophisticated characterisation methods and calculations to explain how this information can be leveraged into the design of more active photocatalysts [15,16]. Kaolinite, with its unique structure and surface properties, may be suitable for use in the production of new photocatalytic materials. The review highlights the latest advancements and outlines the major issues and prospects for enhancing kaolinite-based photocatalysts for large-scale water purification, recommending steps to address global water pollution problems.
Fundamentals of Kaolinite and Photocatalysis
Kaolinite (Al₂Si₂O₅(OH)₄) is a naturally abundant 1:1 layered aluminosilicate clay mineral comprising alternating silica tetrahedral (SiO₄) and alumina octahedral (AlO₆) sheets that are tightly bonded by hydrogen bonds, result- ing in a stable, non-swelling structure. The surface of kaolinite is rich in hydroxyl (-OH) groups. It carries a net negative charge, which enhances its ability to adsorb cationic pollutants and supports the uniform dispersion of semiconductor nanoparticles such as TiO₂ [17,18]. These features are visually depicted in Panel A of the schemat- ic, highlighting the layered arrangement, hydroxyl groups, and negative surface charge.
Kaolinite’s high thermal and chemical stability, along with its moderate specific surface area, make it an excellent support material for photocatalytic applications. The formation of strong interfacial bonds, such as Ti–O–Si and Ti–O–Al, between kaolinite and TiO₂ nanoparticles enhances the stability of the composite and facilitates efficient electron transfer [18,19].
This integration mechanism is illustrated in Panel B, where TiO₂ nanoparticles are anchored on the kaolinite surface, resulting in improved charge separation and reduced recombination.
Photocatalysis is a process in which a semiconductor material absorbs light energy, generating electron-hole pairs (e-/h+) that drive redox reactions capable of degrading organic pollutants. Titanium dioxide (TiO₂) is widely used for this purpose due to its high oxidative power and chemical stability. However, its wide bandgap (~3.2 eV) restricts its activity to the ultraviolet region, and the rapid recombination of photogenerated electron-hole pairs limits its efficiency [20,21].
TiO2 composites with kaolinite overcome these limitations, as they enhance charge separation and light absorp- tion, providing numerous active sites for photocatalytic reactions [10,11]. In ternary composites, such as kaolin- ite/TiO₂/rGO, kaolinite also acts as an electron mediator, further improving charge carrier mobility and reducing recombination loss. These synergistic effects are depicted in Panel C, where light absorption by TiO₂ leads to charge separation, electron migration to kaolinite, pollutant adsorption on the negatively charged surface, and the generation of reactive oxygen species (ROS) for pollutant degradation [22,23].
Comparative studies (see Panel D) have shown that kaolinite/TiO₂ composites outperform pure TiO₂ by exhib- iting enhanced charge separation and synergistic adsorption-photocatalysis, resulting in higher degradation efficiencies for dyes and pharmaceuticals . Moreover, alkaline or acid treatment of kaolinite can create mesopores and enlarge the surface area, thereby improving both adsorption capacity and photocatalytic activity [23,24].
In brief, kaolinite presents a layered structure, surface chemistry, and stability that offer a strong basis for photo- catalytic advanced composites. It is these properties, together with their capacity to form robust interfacial bonds with semiconductors and facilitate efficient charge transfer, that make kaolinite-based hybrid photocatalysts significantly more effective in environmental remediation.
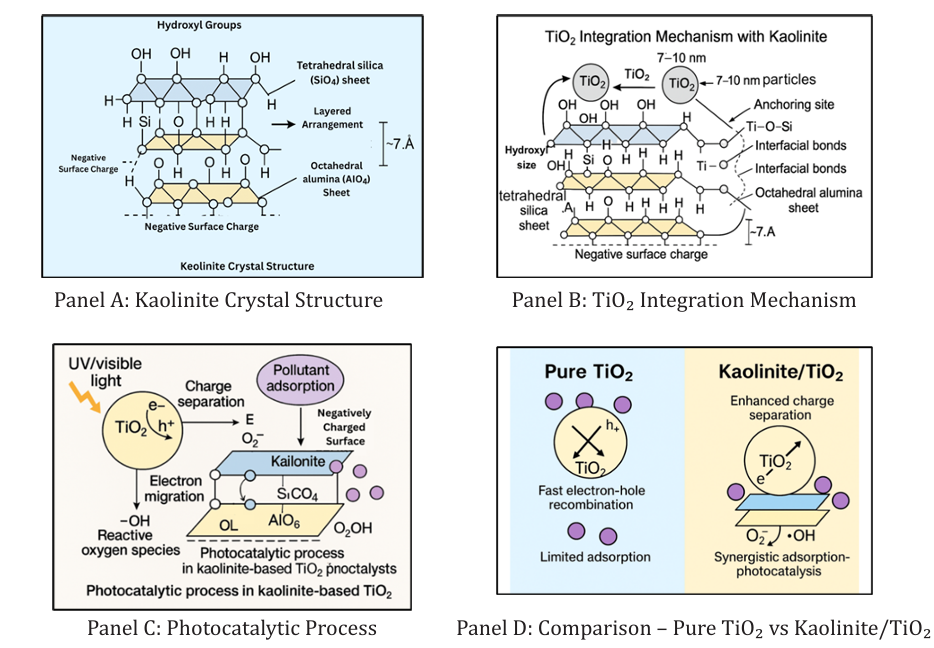 Figure 01: Schematic illustration of kaolinite’s crystal structure and its role in enhancing photocatalytic performance. (A) 1:1 layered structure of kaolinite showing tetrahedral and octahedral sheets with surface hydroxyl groups. (B) Integration mechanism showing Ti–O–Si/Al interfacial bonding and uniform TiO₂ dispersion. (C) Photocatalytic process demonstrating light absorption, charge separation, pollutant adsorption, and ROS generation. (D) Comparative diagram highlighting advantages of kaolinite/TiO₂ composites over pure TiO₂.
Figure 01: Schematic illustration of kaolinite’s crystal structure and its role in enhancing photocatalytic performance. (A) 1:1 layered structure of kaolinite showing tetrahedral and octahedral sheets with surface hydroxyl groups. (B) Integration mechanism showing Ti–O–Si/Al interfacial bonding and uniform TiO₂ dispersion. (C) Photocatalytic process demonstrating light absorption, charge separation, pollutant adsorption, and ROS generation. (D) Comparative diagram highlighting advantages of kaolinite/TiO₂ composites over pure TiO₂.
Materials and Methods
Synthesis and Modification Strategies
Photocatalyst synthesis and modification
The synthesis and surface/structural optimisation of kaolinite-derived photocatalysts are crucial for maximising the photocatalytic activity of the prepared photocatalyst. Physical, chemical, and hybrid methods have been established as various strategies to anchor semiconductors onto kaolinite, enhance interfacial contact, and tailor material properties for specific application [25].
Sol-Gel Method
The sol-gel method is widely used for synthesizing kaolinite-based nanocomposites, particularly TiO₂/kaolinite systems. In this process, a titanium precursor (such as titanium isopropoxide) undergoes hydrolysis and condensation reactions in the presence of kaolinite, followed by drying and calcination. This method enables uniform coating of TiO₂ nanoparticles onto the kaolinite surface, preserving its layered structure while providing precise control over particle size and morphology . For example, TiO₂-kaolinite nanocomposites prepared via the sol-gel method have demonstrated enhanced photocatalytic activity and improved solar light harvesting, with degradation efficiencies for organic dyes, such as Congo red, reaching up to 99% under sunlight [26,27].
Hydrothermal and Solvothermal Methods
Hydrothermal synthesis involves reacting kaolinite and semiconductor precursors in a sealed autoclave at elevated temperatures and pressures. This method produces highly crystalline nanocomposites, promotes strong interfacial bonding, and can generate unique morphologies such as nanotubes or rod-like structures. It is especially effective for synthesizing ZnO/kaolinite and g-C₃N₄/kaolinite composites, as it facilitates the growth of semiconductor nanoparticles on the clay surface while preserving or enhancing its specific surface. The porosity and surface chemistry of the composites can be further adjusted by solvothermal techniques that involve organic solvents [20,28].
Mechanical and Mechanochemical Methods
Mechanical mixing and grinding are simple, scalable techniques for physically combining kaolinite with semiconductor powders. Mechanochemical treatment, which combines mechanical force with chemical reactions, can exfoliate kaolinite layers and promote intimate contact between the clay and semiconductor. This results in enhanced charge transfer and increased active sites. For example, the mechanochemical synthesis of kaolinite/g-C₃N₄ composites results in a sandwich-like structure with enhanced visible-light-driven photocatalytic activity [28,29].
Activation and Calcination
Thermal treatment, or calcination (at temperatures between 600–900°C), produces metakaolin, which has increased surface area and reactivity. Calcination also removes structural water, enhances porosity, and facilitates subsequent acid or base modifications . The calcined kaolinite can then be used as a support for semiconductor deposition or as a precursor for further functionalization [30].
Acid and Base Activation
Chemical activation using acids (e.g., HCl) or bases (e.g., KOH) modifies the surface chemistry of kaolinite by increasing the number of hydroxyl groups and creating additional active sites for adsorption and catalysis. Acid activation can remove octahedral Al³⁺ ions and generate amorphous silica, resulting in surface areas of up to 219 m²/g. These treatments are particularly promising for preparing kaolinite-based adsorbents and catalyst supports [30,31].
Doping and Hybridization
Doping kaolinite-based composites with metals (e.g., Fe, Ag, Ni, Ce) or nonmetals (e.g., N, S) can enhance photocatalytic activity by narrowing the bandgap, increasing visible-light absorption, and improving charge separation . For example, metal-doped kaolinite catalysts synthesized via microwave-assisted hydrothermal methods show significantly improved photocatalytic activity due to higher dispersion and increased oxygen vacancies [31,32]. Hybridizing kaolinite with other semiconductors such as g-C₃N₄, ZnO, or graphene oxide forms heterojunctions that facilitate efficient charge transfer and extend light absorption into the visible range. For instance, kaolinite/TiO₂/graphene composites prepared by sol-gel and hydrothermal methods exhibit enhanced surface area and superior photocatalytic degradation of pharmaceuticals under visible light [20,23].
Self-Assembly and Template Methods
Self-assembly techniques utilize non-covalent interactions to organize nanoparticles or nanosheets on kaolinite, resulting in ordered structures with enhanced photocatalytic properties.
Template-assisted methods utilizing kaolinite as a sacrificial or structural template can produce porous or hollow photocatalysts with high surface areas and tailored pore architectures. These approaches are efficient for creating hierarchical structures that combine the advantages of high surface area with efficient mass transport [31,32].
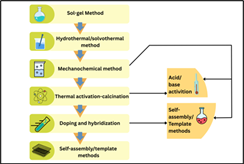 Figure 02: Schematic overview of synthesis and modification strategies for kaolinite-based photocatalysts. (Present Study).
Figure 02: Schematic overview of synthesis and modification strategies for kaolinite-based photocatalysts. (Present Study).
Mechanistic Role of Kaolinite in Photocatalysis
The unique chemical and structural features of kaolinite make photocatalytic systems much more effective. These are the main ways that kaolinite makes photocatalysis work more effectively:
- Structural and Surface Properties: Kaolinite’s 1:1 layered aluminosilicate structure provides a stable, non-swelling framework and a moderate surface area, supporting uniform dispersion of semiconductors and minimizing aggregation. The surface of kaolinite is rich in hydroxyl (-OH) groups, which form strong interfacial bonds (e.g., Ti–O–Si, Ti–O–Al) with semiconductors, enhancing electron transfer and composite stability. Kaolinite’s negatively charged surface promotes electrostatic interactions with cationic pollutants, while its hydroxyl groups act as electron traps, suppressing the recombination of photogenerated electron-hole pairs. In ternary systems (e.g., kaolinite/TiO₂/rGO), kaolinite induces band bending at the semiconductor interface, generating an internal electric field that promotes efficient charge separation [32,33].
- Adsorption-Photocatalysis Synergy: The adsorption properties of kaolinite towards organic dyes and/ or heavy metals can lead to a higher concentration of pollutants near its active sites, resulting in a rapid degradation Studies demonstrate that kaolinite can achieve up to 99% removal of methylene blue and other cationic dyes through electrostatic adsorption, creating high pollutant concentrations at the semiconductor interface for enhanced photocatalytic oxidation. Heavy metal adsorption studies show that kaolinite effectively removes Pb(II), Cd(II), and Cu(II) from aqueous solutions, with removal efficiencies exceeding 90% for most cations [33,34]. The negative surface charge of kaolinite enhances interactions with cationic species through electrostatic attraction, while its point of zero charge (pHpzc ~3.1-3.5) determines the optimal pH range for pollutant adsorption . Acid activation using HCl can remove octahedral Al³⁺ ions and introduce mesopores, increasing surface areas from ~25 m²/g to up to 219 m²/g, thereby further enhancing the adsorption capacity for both organic and inorganic pollutants. This increases the porosity and, consequently, the active sites where pollutants can be bound, facilitating mass transfer in photocatalytic processes [34,35].
- Stability and Reusability: Kaolinite can retain its structure at high temperatures up to 600°C and is thus suitable for high-temperature synthesis routes, including sol-gel and hydrothermal Calcination at 500-900°C converts kaolinite into metakaolinite, which exhibits increased surface reactivity and improved semiconductor anchoring sites due to dehydroxylation and structural reorganization. The thermal stability ensures consistent performance across multiple regeneration cycles and harsh operating conditions (35,36). A well-developed interfacial bond (Ti--O--Si, Ti--O--Al) between kaolinite and semiconductors can contribute to the avoidance of nanoparticle leaching, high stability over extended periods, and a low level of secondary pollution. Kaolinite-based ceramics and membranes exhibit excellent reusability, maintaining a removal efficiency of over 95% after multiple treatment cycles [36,37]. Studies on ceramic pot filters and membrane applications show that kaolinite composites retain structural integrity and adsorption capacity even after extended use in real wastewater treatment (Mogollon-Cuellar et al., 2018; Yanu et al., 2023). The formation of covalent bonds at the kaolinite-semiconductor interface creates stable hybrid structures that resist dissolution and maintain photocatalytic activity over extended periods [37,38].
- Role in Advanced Heterojunctions: Kaolinite plays a crucial role in forming Z-scheme heterojunctions (e.g., g-C₃N₄/TiO₂/kaolinite), acting as an electron bridge to enhance visible-light absorption and charge mobility [38,39]. In kaolinite/TiO₂/rGO composites, kaolinite stabilizes the rGO layers, improving electron transport and reducing recombination losses [39,40]. Table 01 Indicated the roles and functions of each component in kaolinite-based ternary photocatalytic composites, highlighting their structural contributions, photocatalytic mechanisms, and performance enhancements in pollutant degradation application.
Table 01: Indicated the roles and functions of each component in kaolinite-based ternary photocatalytic composites, highlighting their structural contributions, photocatalytic mechanisms, and performance enhancements in pollutant degradation applications.
| Component | Structural Role | Photocatalytic Function | Mechanistic Advantage | Performance Enhancement |
|
Kaolinite |
1:1 layered alumi- nosilicate structure (Al₂Si₂O₅(OH)₄)- Surface hydroxyl groups (-OH) | Electrostatic adsorption of cationic pollutants- Stabilizes metal oxide nanoparticles | Forms Ti–O–Si and Ti–O–Al interfacial bonds with TiO₂- Increases the surface area and adsorption sites | Up to 99% dye degra- dation efficiency- En- hanced light harvest- ing due to kaolinite's surface properties |
|
TiO₂ |
Anatase phase nanoparticles (7–10 nm)- Uniform size distribution | Primary semiconductor photocatalyst- Absorbs UV/visible light | Photogenerated holes remain on TiO₂- Electrons migrate to kaolinite surface to prevent recombination | 2.5× higher activity than pure TiO₂- Enhanced- photocatalytic performance due to kaolinite integration |
| Reduced Graphene Oxide (rGO) | 2D graphene sheets with defect sites- High surface area and conductivity | Electron acceptor and conductor- Facilitates charge transfer in photocatalytic processes | Forms Z-scheme het- erojunctions- Acts as an electron sink to reduce recombination | Reduces recombination losses by 80%- Improved stability and photocatalytic activity compared to pure TiO₂ |
Photocatalytic Applications of Kaolinite-Based Materials
Kaolinite-based composite photocatalysts have found broad applications in environmental remediation, water purification, energyproduction, and antibacterial treatment. Theireffectivenessisrooted in thesynergyofkaolinite’s adsorption capacity, surface chemistry, and its ability to form robust heterojunctions with semiconductors.
Kaolinite-based materials exploit the mineral’s layered structure, surface hydroxyl groups, and strong interfacial bonding with semiconductors to deliver efficient pollutant removal and catalytic activity. Kaolinite-based materials exploit the mineral’s layered structure, surface hydroxyl groups, and strong interfacial bonding with semiconductors to deliver efficient pollutant removal and catalytic activity [39,40].
Kaolinite/TiO₂/rGO and TiO₂/graphene composites achieve up to 99% degradation of MB under visible light, outperforming pure TiO₂ due to enhanced charge separation and pollutant pre-concentration on the kaolinite surface. Graphene–TiO₂ and kaolinite/TiO₂ nanocomposites show high removal efficiency for acid orange seven and Congo red dyes, attributed to improved surface area, π–π stacking, and the generation of reactive oxygen species (ROS) like hydroxyl radicals and superoxide anions [40,41].
Kaolinite/g-C₃N₄, graphene-based, and polyaniline-TiO₂ composites degrade over 90% of pharmaceutical residues under visible light, benefiting from enhanced porosity, charge transfer, and ROS generation. Kaolinite- based adsorbents and composites efficiently remove heavy metals from water, with removal efficiencies exceeding 90% for most cations, due to ion-exchange and surface complexation mechanisms [30,41,42].
Kaolinite’s hydroxyl-rich surface adsorbs cationic pollutants and heavy metals, concentrating them near the active sites of the semiconductor. Acid activation and composite formation introduce mesopores (surface area up to 219 m²/g), boosting adsorption and ROS generation for efficient degradation [20,21,22].
Kaolinite/g-C₃N₄ and TiO₂/kaolinite/graphene composites achieve hydrogen evolution rates up to 288 µmol g⁻¹ h⁻¹, more than double that of pure g-C₃N₄, due to enhanced electron mobility via Ti–O–Si bonds and reduced recombination losses from kaolinite’s negatively charged surface [23,30,42].
- CO₂ Reduction: Calcined kaolinite/TiO₂ composites yield up to 2.5× higher methane production than pure TiO₂ by stabilizing anatase-phase nanoparticles and suppressing electron-hole recombination via interfacial bonding. Non-Precious Co-catalysts: Ni- or Ce-doped kaolinite-based catalysts can replace noble metals, maintaining high hydrogen production at lower Z-scheme heterojunctions (e.g., g-C₃N₄/TiO₂/kaolinite) retain strong redox potentials for CO₂-to-methanol conversion [41,42,43].
- Bacterial Disinfection: Antimicrobial Activity: Kaolinite/ZnO and kaolinite/TiO₂ composites exhibit potent antimicrobial activity, achieving up to 100% inactivation of E. coli within 120 minutes under visible light [44,45,46]. These composites maintain over 95% disinfection efficiency after multiple cycles, with minimal nanoparticle Hydroxyl groups on kaolinite trap holes (h⁺), generating -OH radicals that disrupt bacterial membranes [47,48,49].
Challenges and Future Directions
Current synthesis methods (e.g., sol-gel) are energy-intensive and must be adapted for industrial-scale production. Doping with transition metals (Fe, Ce) or non-metals (N, S) narrows the bandgap, enhancing visible-light absorption. Complex wastewater matrices can reduce efficiency, but hierarchical pore structures and membrane integration improve resilience and long-term performance. Kaolinite-based composites are also being explored for air purification, self-cleaning surfaces, and as precursors for zeolite synthesis in wastewater treatment [50,51,52].
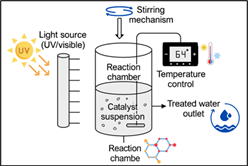 Figure 03: Schematic illustration of a laboratory-scale photocatalytic reactor system used for water treatment experiments (Present Study).
Figure 03: Schematic illustration of a laboratory-scale photocatalytic reactor system used for water treatment experiments (Present Study).
The photocatalytic activity of kaolinite-based composites is promising; however, several key issues need to be addressed to facilitate the rapid implementation of such materials in large-scale environmental remediation and industrial water treatment. A significant problem is the problem of complexity and scalability of synthesis procedures. Multi-step procedures (sol-gel, hydrothermal, or mechanochemical) are frequently needed to create high-performance composites, which need strict control of the reaction conditions, distinctive equipment, and long processing times. Scaling these methods for industrial production is hindered by high energy consumption, reliance on high-purity reagents, and difficulties in maintaining uniform particle size and morphology. Synthesis is additionally complicated by the incorporation of several constituents (e.g., TiO2, rGO, g-C3N4), which raises the cost and restricts its usefulness [53,54,55].
Long-term stability and reusability are crucial for real-world applications. Despite kaolinite’s role in stabilizing nanoparticles, repeated cycles can lead to deactivation due to surface fouling, structural changes, or leaching of active components. For example, kaolinite/TiO₂ composites exhibit efficiency declines after multiple cycles due to the loss of TiO₂ nanoparticles or the accumulation of intermediates. Additionally, potential environmental risks from metal oxide leaching into treated water necessitate rigorous durability and safety assessments [56,57].
Many kaolinite-based photocatalysts remain limited by poor visible-light absorption. While doping (Fe, Ce, N, S) and hybridization with narrow-bandgap materials (g-C₃N₄) extend the activity into the visible spectrum, these strategies often introduce trade-offs such as increased charge recombination or reduced stability. Balancing visible-light responsiveness with charge separation efficiency and durability remains a key challenge [58,59].
Laboratory studies using model contaminants in distilled water cannot replicate the actual complexity of wastewater, which comprises a variety of organic and inorganic species, a wide range of pH levels, and competing ions that inhibit photocatalytic activity. Natural organic matter or high concentrations of chloride and carbonate can scavenge reactive oxygen species (ROS) or block active sites, thereby reducing degradation efficiency. Extensive testing in realistic conditions is a prerequisite for field deployment [60,61].
Although these aspects have been improved, the specifics of interfacial electron transfer, band alignment, and ROS generation in kaolinite-based composites remain unknown. Advanced characterization techniques such as in-situ spectroscopy and computational modelling are needed to elucidate charge carrier dynamics at kaolinite– semiconductor interfaces. This knowledge is critical for the rational design of optimized photocatalysts [60,63].
While kaolinite is abundant and low-cost, the sustainability of these composites depends on synthesis methods, dopant toxicity, and energy requirements. Noble metal co-catalysts (e.g., Pt) increase costs, while nanoparticle leaching and end-of-life disposal pose environmental risks. Life cycle assessments (LCAs) and circular economy approaches are urgently needed to evaluate and improve sustainability [64,65,66] (Figure, Table 2).
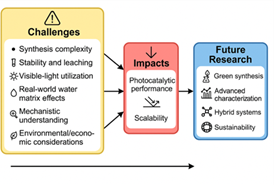 Figure 04: Flowchart illustrating the interconnections between key challenges, their impacts, and future research directions for kaolinite-based hybrid photocatalysts.
Figure 04: Flowchart illustrating the interconnections between key challenges, their impacts, and future research directions for kaolinite-based hybrid photocatalysts.
Table 02: Key research priorities and future opportunities for advancing kaolinite-based photocatalysts multifunctional environmental remediation technologies.
| Research Area | Current Status/Limitation | Future Opportunity/Priority | Expected Impact |
| Scalable & Green Synthesis | Energy-intensive, muti-step lab methods; limited scalability | Develop low-energy, green, and continuous-flow synthesis methods for large-scale production | Cost reduction, industrial adoption |
| Visible-Light Utilization | Most composites remain UV-dependent; low solar efficiency | Bandgap engineering via doping (Fe, Ce, N, S) and hybridization with narrow-bandgap semiconductors | Enhanced solar-driven photocatalysis |
|
Mechanistic Understanding |
Incomplete knowledge of charge transfer and interfacial electron dynamics in composites | In-situ spectroscopy (TRPL, EPR); DFT modeling for better understanding of electron and hole behavior in composites | Rational catalyst design, higher efficiency |
| Hybrid & Multifunctional Systems | Mostly binary/ternary composites; limited multifunc- tional systems for energy and pollutant removal | Develop membranes, biohybrids, and energy storage systems integrated with photocatalysts | Simultaneous pollutant removal & energy production |
|
Real-World Performance |
Lab tests use model pollutants; real wastewater matrices affect performance due to complex composition and ions | Test in real wastewater matrices; hierarchical pore structures for enhanced resilience and performance in practical settings | Reliable operation in practical conditions |
Conclusions
Hybrid photocatalysts composed of kaolinite, particularly those incorporating TiO₂ and reduced graphene oxide (rGO), have shown great promise in degrading dyes, pharmaceuticals, and other recalcitrant water pollutants. The unique layered structure and surface chemistry of kaolinite enable efficient pollutant adsorption and uniform dispersion of semiconductor nanoparticles, leading to enhanced charge separation and photocatalytic activity under visible light. More recently, the functional performance of these composites has been further enhanced by various synthesis routes, including sol-gel, hydrothermal, and mechanochemical routes, and has been utilized in the removal of pollutants, hydrogen production, and the disinfection of bacteria. However, several challenges remain before these materials can be widely applied in real-world scenarios. Key limitations include the complexity and scalability of synthesis methods, the long-term stability and reusability of the materials, control over nanoparticle leaching, limited utilization of visible light, and a deeper understanding of charge transfer at interfaces. Moreover, it is necessary to test under real water matrix conditions and conduct a comprehensive environmental and economic analysis to verify the sustainability of these photocatalysts.
References
1. Chen, M., Han, Y.-R., Hu, J.-X., Liu, Y.-J., & Huang, B. (2023). Tolypocladium rhizomatum sp. nov.: an endophytic species isolated from the rhizome of Polygonatum cyrtonema. Phytotaxa, 3, 201–210. https://doi.org/10.11646/phytotaxa.607.1.10
2. Liu, R., Fan, Y., Guo, R., Dong, X., & Ma, X. (2024). Preparation of Coal Kaolinite Composites and Study of Adsorption Properties. Coal Science and Technology, 52(12),311-323. https://doi.org/10.12438/cst.2023-1632.
3. Hasan, A.K.M.M., Dey, S.C., & Rahman, M.M. (2020). A kaolinite//ZnO-based novel ternary composite for photocatalytic degradation of anionic azo dyes. Buletin of Materials Sciences, 43 (2), 27-35.https://doi.org/10.1007/s12034-0191964-4
4. Mustapha, J.O., Tijani, M.M., Ndamitso, A.S., Abdulkareem, D.T., & Shuaib, A. K., Mohammed, H. (2021). Adsorptive removal of pollutants from industrial wastewater using mesoporous kaolin and kaolin/TiO2 nanoadsorbents. Environmental Nanotechnology, Monitoring & Management, 15 (3), 60-80. https://doi.org/10.1016/j.enmm.2020.100414.
5. Ayanda, S. O., Kehinde, O., Sodeinde, P. O., Okolo, P., Ayomitide, A. (2018). Adsorptive Behavior of Kaolin for Amido Black Dye in Aqueous Solution. Oriental Journal of Chemistry, 34(3), 1233-1239. http://dx.doi.org/10.13005/ojc/340305
6. Septiansyah, S. I., Afandi, I. H., & Dirtami, R. T. (2022). Activation of Kaolin Minerals from Ketapang Regency as Cu Metal Adsorbent Material. EKSPLORIUM, 43(2), 103–110. https://doi.org/10.17146/eksplorium.2022.43.2.5802.
7. Shaban, M., Hassouna, M.E.M., Nasief, F.M., AbuKhadra, M.R. 2017 Adsorption properties of kaolinite-based nanocomposites for Fe and Mn pollutants from aqueous solutions and raw ground water: kinetics and equilibrium studies. Environmental Sciences and Pollution Research International, 24(29), 22954-22966. https://doi:10.1007/s11356-017-9942-0
8. Zen, S., Zohra, F., and Berrichi, F. (2016). Absorption of tannery anionic dyes by modified kaolin from aqueous solution. Desalination and Water Treatment,57 (13), 57, Issue 13,6024-6032, https://doi.org/10.1080/19443994.2014.981218.
9. Aloulou, H., Aloulou, W., Duplay, J., Baklouti, L., Dammak, L., & Ben Amar, R. (2022). Development of Ultrafiltration Kaolin Membranes over Sand and Zeolite Supports for the Treatment of Electroplating Wastewater. Membranes, 12(11), 10661080. https://doi.org/10.3390/membranes12111066.
10. Detellier C. (2018). Functional Kaolinite. The Chemical Records, 18(7-8), 868-877. https://doi: 10.1002/tcr.201700072.
11. Juliana, O., Fatima, B., Ibrahim, H., Abdullahi, S., Argungu, F., & David, O. (2023). Development and optimization of hydroxyapatite/kaolin-based ceramic materials as potential adsorbents for water purification. Environmental Advances, 13(2), 67-80. https://doi.org/10.1016/j.envadv.2023.100419.
12. Kong, E. D. H., Chau, J. H. F., Lai, C. W., Khe, C. S., Sharma, G., Kumar, A., Siengchin, S., & Sanjay, M. R. (2022). GO/TiO2 Related Nanocomposites as Photocatalysts for Pollutant Removal in Wastewater Treatment. Nanomaterials, 12(19), 3536- 3550. https://doi.org/10.3390/nano12193536
13. Lata, N. P., Hussain, M. S., Mamun, A. H., Rashid, T. U., Shamsuddin, S. (2024). Fabrication and synergistically enhanced photocatalytic activity of ternary kaolinite, TiO2, and Al2O3 (K65T30A5) nanocomposite for visible-light-induced degradation of methylene blue and remazol red dye. Heliyon, 10(8), 50-70. https://doi.org/10.1016/j.heliyon.2024.e29255.
14. Posa, V. R., Annavaram, V., Somala, A. R. (2016). Fabrication of graphene–TiO2 nanocomposite with improved photocatalytic degradation for acid orange 7 dye under solar light irradiation. Bulletin of Material Sciences, 39 (2), 759–767.https://doi.org/10.1007/s12034-016-1215-x.
15. Potbhare A K, Aziz, S. K., & Tayyub, M. M. (2024). Bioinspired graphene-based metal oxide nanocomposites for photo catalytic and electrochemical performances: an updated review. Nanoscale Advances, 6(10), 2539-2568. https://doi.org/10.1039/D3NA01071F.
16. Sagita, C. P., Nulandaya, L., & Kurniawan, Y. S. (2021). Efficient and Low-Cost Removal of Methylene Blue using Activated Natural.
17. Kaolinite Material. Journal of Multidisciplinary Applied Natural Science, 1(2), 69–77. https://doi.org/10.47352/jmans.v1i2.80
18. Yanu, C. , Nguiamba, N. , Sieliechi, J., & Ngassoum, M. (2023) Elaboration of Ceramic Pot Filter from Kaolinite (Cameroon Clay) for the Elimination of Suspended Particles from Domestic Drinking Water. Journal of Materials Science and Chemical Engineering, 11 (3), 43-60. https://doi.org/10.4236/msce.2023.118004.
19. Alkhabbas, M., Odeh, F., Alzughoul, K., Afaneh, R., & Alahmad, W. (2023). Jordanian Kaolinite with TiO2 for Improving Solar Light Harvesting Used in Dye Removal. Molecules, 28(3), 989-999. https://doi.org/10.3390/molecules28030989
20. Abdessamad, B., Fatima, Z., Brahim, A., Toussaint, N. k., Saad, A. Y., Redouane, B., Dani, A., Rachid, B., & Ouammou, M. (2021). Optimization of phosphate/kaolinite microfiltration membrane using Box–Behnken design for treatment of industrial wastewater. Journal of Environmental Chemical Engineering, 9 (1), 104-120. https://doi.org/10.1016/j. jece.2020.104972.
21. Bodzek, M., Krystyna, K., Anna, K. (2021). Nano-photocatalysis in water and wastewater treatment. Desalination and Water Treatment, 243 (2), 51-74. https://doi.org/10.5004/dwt.2021.27867.
22. Cheng, H., Zhou, Y., Qinfu, L. (2019). Kaolinite Nanomaterials: Preparation, Properties and Functional Applications, Editor(s): Aiqin Wang, Wenbo Wang, In Micro and Nano Technologies, Nanomaterials from Clay Minerals, Elsevier,Pages 285-334, https://doi.org/10.1016/B978-0-12-814533-3.00006-5.
23. Dey, A., & Gogate, A. H. (2021). Nanocomposite photocatalysts-based wastewater treatment. Editor(s): Bharat Bhanvase, Shirish Sonawane, Vijay Pawade, Aniruddha Pandit, In Micro and Nano Technologies, Handbook of Nanomaterials for Wastewater Treatment, Elsevier, Pages 779-809. https://doi.org/10.1016/B978-0-12-821496-1.00022-2.
24. LI, G., Liu J., & Zhou, T. (2021). High efficient photodegradation of organic dyes by TiO2 /graphene composite under visible light radiation. Desalination and Water Treatment, 213 (2), 319-327. https://doi.org/10.5004/dwt.2021.26717
25. Mohamed, F. M., EL-AASSAR, M. R., & Abdullah, A. M. (2024). Novel method of poly aluminum chloride extraction from kaolin and its application for wastewater treatment. Desalination and Water Treatment, 317(2), 450-465. 450-465. https://doi.org/10.5004/dwt.2021.26717
26. Anku, W.W., Kiarii, E. M., & Sharma, S, H. (2019). Photocatalytic Degradation of Pharmaceuticals Using Graphene Based Materials. A New Generation Material Graphene: Applications in Water Technology, 213(2), 187-208. https://doi.org/10.5004/dwt.2021.26717
27. Avornyo A. K., Hasan, S.W., Banat, F., & Chrysikopoulos C V. (2024). Preparation, characterization, and applications of kaolin/kaolin-based composite membranes in oily wastewater treatment: Recent developments, challenges, and opportunities. Jornal of Environmental Management, 370 (3), 122-130.https://doi.org/10.1016/j.jenvman.2024.122800
28. Jangid, N. K., Jadoun, S., & Yadav, A. (2020). Polyaniline-TiO2-based photocatalysts for dyes degradation. Polymer Bulletin, 78(8), 4743-77. https://doi.org/10.1007/s00289-020-03318-w
29. Kamal, S., Kamal, A., Shahzad, T., Ismat, D., et al. (2017). Potential of kaolinite as adsorbent to remove anionic surfactant from simulated industrial wastewater. Desalination and Water Treatment, 88, 85-92. https://doi.org/10.5004/dwt.2017.21342
30. Kumaran, V., Sudhagar, P., Konga, A. K., & Ponniah, G. (2020). Photocatalytic degradation of synthetic organic reactivedye wastewater using GO-TiO2 nanocomposite. Polish Journal of Environmental Studies, 29(2), 1683-1690.https://doi.org/10.15244/pjoes/109027
31. Azeez, S. O., Saheed, I. O., Shina, S. S. (2022). Preparation of TiO2-activated kaolinite composite for photocatalytic degradation of rhodamine B dye. Bulletin of the Chemical Society of Ethiopia, 36(1), 13-24. https://doi.org/10.4314/bcse.v36i1.2
32. Perez-A lvarez, J., Solis, D., Romero, S., Escobar-Alarcon, L. (2014). Photocatalytic degradation of malachite green dye and pharmaceuticals using Co:TiO2 thin films. Advanced Materials Research, 976, 212-216. https://doi.org/10.4028/www.scientific.net/AMR.976.212
33. Ramesh, N., Lai, C. W., Johan, M. R. B., Mousavi, S. M., Badruddin, I. A., Kumar, A., Sharma, G., Gapsari, F. (2024). Progress in photocatalytic degradation of industrial organic dye by utilizing the silver doped titanium dioxide nanocomposite.Heliyon, 10(24), e40998. https://doi.org/10.1016/j.heliyon.2024.e40998
34. Xu, H., Liu, J., Chen, P., & Zhang, R. (2018). Preparation of magnetic kaolinite nanotubes for the removal of methylene blue from aqueous solution. Journal of Inorganic and Organometallic Polymers and Materials, 28(3), 461-470. https://doi.org/10.1007/s10904-017-0728-0
35. Barrera-Díaz, C. E., Lugo-Lugo, V., & Bilyeu, B. (2012). A review of chemical, electrochemical and biological methods for aqueous Cr(VI) reduction. Journal of Hazardous Materials, 229, 1-14. https://doi.org/10.1016/j.jhazmat.2012.04.054
36. Egirani, D. E., Latif, M. T., Wessey, N., & Acharyjee, S. (2019). Synthesis and characterization of kaolinite coated with copper oxide and its effect on the removal of aqueous Lead(II) ions. Applied Water Science, 9(4), 1-13. https://doi.org/10.1007/s13201-019-0989-6
37. Giovannetti, R., Rommozzi, E., Zannotti, M., & D’Amato, C. A. (2017). Recent advances in graphene based TiO2 nano composites (GTiO2Ns) for photocatalytic degradation of synthetic dyes. Catalysts, 7(10), 305. https://doi.org/10.3390/catal7100305
38. Kholodko, Y., Bondarieva, A., Tobilko, V., Glukhovskiy, V. (2022). Synthesis and characterization of kaolinite-based granular adsorbents for the removal of Cu(II), Cd(II), Co(II), Zn(II), and Cr(VI)from contaminated water. Eastern-European Journal of Enterprise Technologies, 4(10), 6-13. https://doi.org/10.15587/1729-4061.2022.262994
39. Sari, Y., Gareso, P. L., & Tahir, D. (2025). Review: Influence of synthesis methods and performance of rare earth doped TiO2 photocatalysts in degrading dye effluents. International Journal of Environmental Science and Technology, 22(3),1975-1994. https://doi.org/10.1007/s13762-024-05879-z
40. Sarma, G. K., Gupta, S. S., & Bhattacharyya, K. G. (2019). Removal of hazardous basic dyes from aqueous solution by adsorption onto kaolinite and acid-treated kaolinite: Kinetics, isotherm, and mechanistic study. SN Applied Sciences,1(3), 1-13. https://doi.org/10.1007/s42452-019-0216-y.
41. Sultana, M., Mondal, A., Islam, S., Khatun, A., Rahman, M. H., Chakraborty, A. K., Rahman, M. S., & Nur, A. S. M. (2023). Strategic development of metal doped TiO2 photocatalysts for enhanced dye degradation activity under UV-Vis irradiation: A review. Current Research in Green and Sustainable Chemistry, 7, 100383.https://doi.org/10.1016/j.crgsc.2023.100383.
42. Bai, C. H., Zheng, S. L., Sun, Z., Wen, M. (2010). TiO2/Kaolinite photocatalytic material of Fe chemical doping and Fe2O3 heat-banding and its mechanism analysis. Advanced Materials Research, 178, 324-329. https://doi.org/10.4028/www.scientific.net/AMR.178.324
43. Kholodko, Y., Bondarieva, A., Tobilko, V., Glukhovskiy, V. (2022). Synthesis and characterization of kaolinite-based granular adsorbents for the removal of Cu(II), Cd(II), Co(II), Zn(II), and Cr(VI) from contaminated water. Eastern-European Journal of Enterprise Technologies, 4(10), 6-13. https://doi.org/10.15587/1729-4061.2022.262994
44. Barbosa, A. D. S., Rodrigues, M., & Rodrigues, D. P. A. (2021). Use of kaolin as a potential low-cost adsorbent for the removal of reactive blue BF-5G dye. Research Society and Development, 10(12), e13101220035. https://doi.org/10.33448/rsd-v10i12.20035
45. Karunadasa, K. S. P., Wijekoon, A. S. K., & Manoratne, C. (2023). TiO2-kaolinite composite photocatalyst for industrial organic waste decontamination. Next Materials, 3(2), 100065. https://doi.org/10.1016/j.nxmate.2023.100065
46. Krakowiak, R., Musiał, J., Bakun, P., Goslinski, T. (2021). Titanium dioxide-based photocatalysts for degradation of emerging contaminants including pharmaceutical pollutants. Applied Sciences, 11(18), 8674.https://doi.org/10.3390/app11188674
47. Bai, L., Kang, P., Chen, H., & Wang, Z. (2018). TiO2 nanoparticles assembled on kaolinites with different morphologies for efficient photocatalytic performance. Scientific Reports, 8(1), 29563. https://doi.org/10.1038/s41598-018-29563-8
48. Paumo, H. K., Dalhatou, S., Katata-Seru, L., & Bahadur, I. (2021). TiO2 assisted photocatalysts for degradation of emerging organic pollutants in water and wastewater. Journal of Molecular Liquids, 331, 115458. https://doi.org/10.1016/j.molliq.2021.115458
49. Pimneva, L. A., & Zagorskaya, A. (2021). The ion-exchange properties of kaolinite in the practice of natural water purification. Ecological Engineering & Environment, 22(2), 87-91. https://doi.org/10.12912/27197050/133381
50. Rivera-Ortiz, S. F., Salazar-Ayala, K. A., & Pena-Rodríguez, G. (2018). Treatment of subterranean mining water throughfiltration using ceramic bilayer membranes based on recycled diatomites and kaolin. Contemporary Engineering Sciences, 11(89), 4437-4446. https://doi.org/10.12988/ces.2018.88480
51. Yu, L., Xu, W., Liu, H., & Bao, Y. (2022). Titanium dioxide–reduced graphene oxide composites for photocatalytic degra dation of dyes in water. Catalysts, 12(11), 1340. https://doi.org/10.3390/catal12111340
52. Aragao, D. M., Arguelho, M. L. P. M., Oliveira Prado, C. M., & Alves, J. P. H. (2015). Use of natural kaolinite clay as an adsorbent to remove Pb(II), Cd(II), and Cu(II) from aqueous solution. Materials Science Forum, 805, 581-584.https://doi.org/10.4028/www.scientific.net/MSF.805.581
53. Bondarieva, A., Yaichenia, I., Zahorodniuk, N., Tobilko, V., & Pavlenko, V. (2022). Water purification from cationic organic dyes using kaolin-based ceramic materials. Technology Audit and Production Reserves, 2(64), 10-16.https://doi.org/10.15587/2706-5448.2022.254584
54. Mogollon-Cuellar, Z. Y., Silva-Rivero, Y. I. N., & Pena-Rodríguez, G. (2018). Water treatment using porous ceramics based on recycled diatomite and kaolin. Contemporary Engineering Sciences, 11(75), 3729-3738.https://doi.org/10.12988/ces.2018.88366
55. Kamel, M. M., Ibrahim, M. A., Ismaiel, A., & El-Motaleeb, M. A. (2004). Adsorption of some heavy metal ions from aqueous solutions by using kaolinite clay. Environmental Engineering Science, 21(3), 293-300.
56. Reyes, C. A. R., & Fiallo, L. Y. V. (2011). Application of Illite- and Kaolinite-Rich Clays in the Synthesis of Zeolites for Wastewater Treatment. Earth and Environmental Sciences, 2(64), 10-16. https://doi.org/10.5772/25895
57. Ramísio, P. J., & Vieira, J. M. P. (2010). Evaluation of Zn, Cu, and Pb sorption-desorption phenomena in kaolinite-sand media filtration pilot scale installation. Alliance for Global Sustainability, 2010, Conference Paper. Springer.https://doi.org/10.1007/978-90-481-3043-6_34
58. Shahmohammadi-Kalalagh, S., Babazadeh, H., Nazemi, A. H., & Manshouri, M. (2010). Isotherm and Kinetic Studies on Adsorption of Pb, Zn, and Cu by Kaolinite. Journal of Environmental Management, 91(11), 2075-2082.
59. Prasad, M.S., Reid, K.J., Murray, H.H. (1991). Kaolin: processing, properties and applications. Applied Clay Science, 6(2), 87-119. https://doi.org/10.1016/0169-1317(91)90001-P
60. Alaqarbeh, M. M., Shammout, M. M., & Awwad, A. M. (2020). Nano platelets kaolin for the adsorption of toxic metal ions in the environment. Zenodo. https://doi.org/10.5281/zenodo.3361011
61. Lei, S., Gong, W., Bai, C., Qu, Y., Gu, Y., Xiong, B., & Wang, C. (2006). Preparation of TiO₂/Kaolinite nanocomposite and its photocatalytical activity. Journal of Wuhan University of Technology-Mater. Sci. Ed., 21, 12-15. https://doi.org/10.1007/BF02841194
62. El Berrichi, F. Z., & Zen, S. (2014). Removal of anionic dyes from aqueous solutions using local activated kaolins as adsorbers. Proceedings of the 2014 International Conference on Power Systems, Energy, Environment (pp. 437-444).International Association for Science and Technology.
63. Oladoja, N.A., & Imohimi, A.(2005). Studies on the use of fortified kaolinitic soil-clay in industrial wastewater treatment. Water Quality Research Journal, 40(4), 297-305. https://doi.org/10.2166/wqrj.2005.054
64. Chowdhury, Z., Samrat, S., & Barma, S. (2024). Synthesis, characterization and modification of cost-effective kaolinsupported hydrothermally fabricated mesoporous silica membrane. Journal of Physics:Conference Series, 2818(012030).https://doi.org/10.1088/1742-6596/2818/1/012030
65. Gupta, S. S., & Bhattacharyya, K. G. (2012). Using aqueous kaolinite suspension as a medium for removing phosphate from water. Adsorption Science & Technology, 30(6), 533-548. https://doi.org/10.1260/0263-6174.30.6.533
66. Liu, R., Fan, Y., Guo, R., Dong, X., & Ma, X. (2024). Preparation of coal kaolinite composites and study of adsorption properties. Coal Science and Technology, 52(12), 311-323. https://doi.org/10.12438/cst.2023-1632
This article licensed under the Creative Commons Attribution 4.0 International License CC-BY 4.0., which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are properly credited.
