NL Journal of Veterinary and Animal Nutrition
Distribution and Mapping of Mosquito Vectors Breeding Habitats in Some Areas of Jos North, Plateau State, Nigeria
Author(s) : Njila H L, Ossai H C, Ombugadu A, Polycarp I A, Valentine C C, John A D.
Abstract
Mosquitoes are primary vectors of infectious agents affecting humans and animals. This study aimed to identify and map mosquito breeding habitats in Jos North Local Government Area, Plateau State. Mosquito breeding habitats were surveyed between October and November 2023 using the dipping method and Global Positioning System (GPS). A total of 3,009 mosquito larvae, comprising 2 genera and 2 species, were collected. Culex quinquefasciatus (63.64%) was the most abundant species, followed by Anopheles gambiae (36.36%). No significant difference in mosquito larvae abundance was found across locations (P = 0.4098). However, significant differences were observed in Anopheles larvae abundance across locations (P = 0.01288). Eight breeding habitats were identified and mapped, with riverbank/bed habitats showing the highest abundance of mosquito larvae. The study’s findings highlight the importance of understanding breeding habitats and mapping malaria vectors to inform effective control programs and mitigate the risk of mosquito-borne infections. Keywords: Mosquitoes, Mapping, Breeding habitats, Jos North, Plateau State.
Introduction
Mapping the spatial distribution of mosquito breeding habitats is crucial for effective deployment of control practices [1]. Geospatial mapping, using remote sensing, offers the potential to identify breeding habitats over large areas, which is difficult to achieve through conventional ground surveys [2]. The integration of Geographic Information System (GIS) and Remote Sensing (RS) technologies has become increasingly important in studying the spatial and temporal patterns of vector distribution [3]. This research aims to combine GIS/RS environmental and entomological data to map the breeding habitats of mosquito species. Mosquitoes are primary vectors of infectious agents in humans and animals, transmitting parasites that cause malaria, filariasis, and various viruses (e.g., dengue, yellow fever, and encephalitis) [4,5]. While many studies have focused on the epidemiological role of mosquitoes, researchers have also highlighted their ecological importance, particularly in providing a food source for fish and aquatic invertebrates [6]. Mosquitoes inhabit various larval habitats, including lakes, pools, stagnant water in artificial containers, and tree holes [7].
However, many habitats are species-specific, requiring specific aquatic parameters [8]. In peri-urban areas, artificial recipients serve as crucial sites for mosquito life cycles, including oviposition, larvae and pupae development, and adult emergence [9]. Studying anopheline larvae is essential for understanding malaria transmission risk [10]. Source reduction through modification of larval habitats is critical for malaria eradication efforts. Effective management of larval habitats can suppress vector and malaria transmission. However, anopheline larval ecology is limited, partly due to the challenges involved in larval sampling from aquatic habitats [11].
Recent advancements in geographic positioning system (GPS) and GIS technologies offer alternative approaches to traditional vector surveillance [10]. Remote sensing (RS) and GIS are valuable tools for assessing the spatial epidemiology of vector-borne diseases and analyzing human risk of infection [12]. This study aims to identify and map mosquito breeding habitats using GPS in some areas of Jos North L.G.A, Plateau State.
Materials and Methods
Study Area
Jos North Local Government Area is situated between Latitude 9°05’N and Longitude 8°05’4”E. The area experiences a high rainfall of approximately 1351 mm, with a temperature range of 20°C (minimum) to 28°C (maximum). Due to its elevated altitude compared to surrounding areas at the same latitude, the relative humidity is relatively low during the dry season and increases during the rainy season. This study was conducted in nineteen selected areas of Jos North Local Government Area, which include: 3 Container, Alheri, Angwan Rukuba, Angwan Rimi, Angwan Suya, Apata, Area command, Atili, Chorbe, Etobaba, Faringada, Gada Biyu, Lamingo, Mazah, Rafin Karfe, Russau, Unijos Permanent site, West of mines, and Zololo (Figure 1).
Sampling Period
A survey of mosquito breeding habitats was conducted between October and November 2023. The surveys were carried out during the early hours of the day, from 6:30 am to 1:00 pm, on a daily basis. To ensure accuracy and comprehensiveness, most breeding habitats were visited twice a week during this period.
Collection of Entomological Data
Mosquito breeding habitats were surveyed using the dipping sampling method. To minimize disruption, the dipper was gently lowered into the water at an angle of approximately 45°. The top layer of water was skimmed, allowing nearby larvae to flow into the dipper. The dipper was then carefully raised from the water to prevent spillage. In cases where breeding habitats featured emerging vegetation, the water was gently disturbed to prompt larvae to swim downwards. Vegetation was then removed using the dipper to create a clear sampling area. Collected larvae were transferred to a white plastic bucket and transported to the laboratory for taxonomic identification. Identification was conducted using the keys of Harbach [13], Harbach [14], and Glick [15].
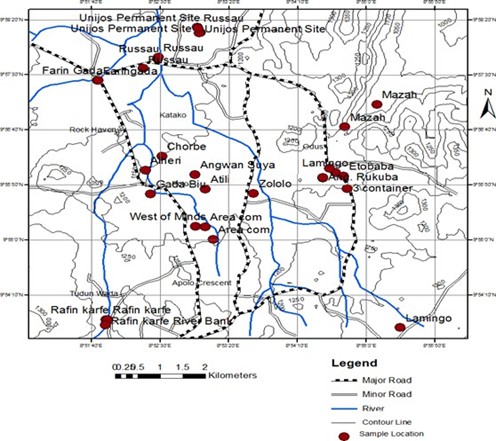
Figure 1: Map of the study areas in Jos North LGA, Plateau State, Nigeria.
Determination of Mosquito Breeding Habitats
Breeding habitats positive for mosquitoes were identified and described based on their characteristic features. The habitats were categorized into the following types: Tyre tracks, Swampy areas, Ditches, Riverbank/bed. Wet-lands (featuring grasses, rushes, reeds, Typha, sedges, and other herbaceous plants in shallow water), Gutters, Temporary Water Bodies and Hoof prints. These habitat characteristics provided a framework for understanding the diverse environments that support mosquito breeding.
Mapping of Mosquito Breeding Habitats
The coordinates of habitats containing mosquito larvae were recorded using a hand-held Global Positioning System (GPS) device (Garmin GPSMAP 60CSx). The GPS data were extracted using Map Source software and subsequently integrated into a Geographic Information System (GIS) database using ArcGIS 9.3 software. This integration enabled the quantification of spatial heterogeneity in the associated area and provided a graphical visualization of mosquito-breeding sites. Specifically, it allowed for the identification of zones with high larval abundance and their correlation with nearby water bodies. Raster images of the site were overlaid with feature layers and entomological data in the GIS database, facilitating a comprehensive analysis of the spatial distribution of mosquito breeding habitats.
Statistical Analyses
Data analysis was performed using R version 3.2.2. One-way analysis of variance (ANOVA) was employed to compare the abundance of mosquito larvae across different locations and habitat types. Additionally, Welch’s two-sample t-test was used to compare the mean abundance of larvae between mosquito species. Statistical significance was set at P < 0.05.
Results
The species composition of mosquito larvae collected during this study is presented in Table 1. A total of 3,009 mosquito larvae were collected, belonging to 2 genera and 2 species. The abundance of larvae differed significantly between mosquito species (t = -2.4641, df = 48.47, P = 0.01733) (Figure 2). Culex quinquefasciatus was the most abundant species, accounting for 63.64% (1,915 individuals) of the total larvae collected. Anopheles gambiae was the least abundant species, representing 36.36% (1,094 individuals) of the total larvae collected. No significant difference was found in the abundance of mosquito larvae across different locations (F13 = 1.144, Adjusted R2 = 0.0771, P = 0.4098) (Figure 3). However, Russau had the highest abundance of mosquito larvae, with 508 individuals (16.88%), while 3 Container had the lowest abundance, with 17 individuals (0.56%). A significant difference was found in the abundance of Anopheles larvae across different locations (F13 = 3.504, Adjusted R2 = 0.5925, P = 0.01288) (Figure 4). Rafin Karfe had the highest abundance of Anopheles larvae, while 3 Container and West of Mine areas had the lowest abundance. In contrast, no significant difference was found in the abundance of Culex larvae across different locations (F13 = 1.013, Adjusted R2 = 0.007557, P = 0.5012) (Figure 5). Russau had the highest abundance of Culex larvae, while 3 Container had the lowest abundance.
A total of 8 breeding habitats of mosquito species were identified across the 19 study areas. The abundance of mosquito larvae varied significantly across these breeding habitats (F24 = 3.79, Adjusted R2 = 0.3865, P = 0.006595) (Figure 6). Riverbank breeding habitats yielded the highest abundance of mosquito larvae, while tyre track breeding habitats had the lowest abundance. The abundance of Anopheles larvae did not differ significantly across breeding habitats (F24 = 1.345, Adjusted R2 = 0.07231, P = 0.273) (Figure 7). However, riverbank breeding habitats had the highest abundance of Anopheles larvae, while tyre track breeding habitats had the lowest. Similarly, the abundance of Culex larvae did not differ significantly across breeding habitats (F24 = 2.22, Adjusted R2 = 0.2159, P = 0.06874) (Figure 8). Riverbank breeding habitats had the highest abundance of Culex larvae, while tyre track breeding habitats had the lowest. A density map (Figure 9) represented the 19 study areas and their respective mosquito breeding habitats, with Anopheles species indicated by red numbers and Culex species indicated by blue numbers.
Table 1: Species Composition of Mosquito larvae in some areas of Jos North LGA, Plateau State.
|
Area |
Mosquito Larvae |
Total (%) |
|
| Anopheles gambiae | Culex quinquefasciatus | ||
| 3 Container | 7 | 10 | 17 (0.56) |
| Alheri | 60 | 112 | 172 (5.72) |
| Angwan Rimi | 60 | 89 | 149 (4.95) |
| Angwan Rukuba | 102 | 358 | 460 (15.29) |
| Angwan Suya | 30 | 98 | 128 (4.25) |
| Apata | 13 | 43 | 56 (1.86) |
| Area command | 25 | 73 | 98 (3.26) |
| Atili | 8 | 30 | 38 (1.26) |
| Chorbe | 32 | 63 | 95 (3.16) |
| Etobaba | 10 | 27 | 37 (1.23) |
| Faringada | 28 | 40 | 68 (2.26) |
| Gada Biyu | 46 | 96 | 142 (4.72) |
| Lamingo | 41 | 15 | 56 (1.86) |
| Mazah | 146 | 124 | 270 (8.97) |
| Rafin Karfe | 267 | 127 | 394 (13.09) |
| Russau | 59 | 449 | 508 (16.88) |
| Unijos Permanent Site | 138 | 65 | 203 (6.75) |
| West of mine | 7 | 24 | 31 (1.03) |
| Zololo | 15 | 72 | 87 (2.89) |
| Total | 1094 | 1915 | 3009 |
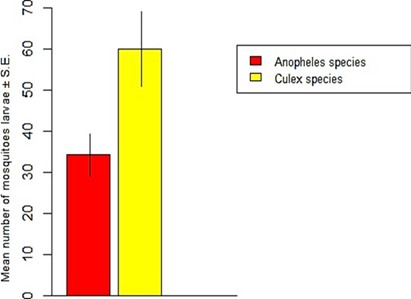 Figure 2: Abundance of larvae in relation to mosquito species.
Figure 2: Abundance of larvae in relation to mosquito species.
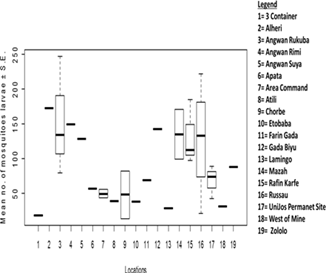 Figure 3: Abundance of mosquito larvae in relation to locations.
Figure 3: Abundance of mosquito larvae in relation to locations.
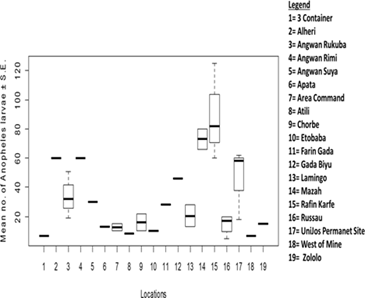 Figure 4: Abundance of Anopheles larvae in relation to locations.
Figure 4: Abundance of Anopheles larvae in relation to locations.
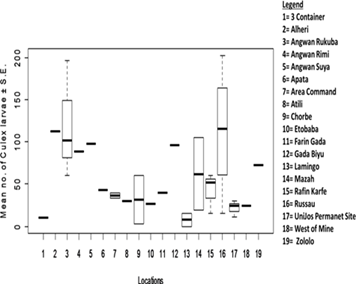 Figure 5: Abundance of Culex larvae in relation to locations.
Figure 5: Abundance of Culex larvae in relation to locations.
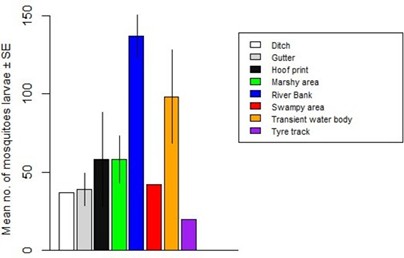 Figure 6: Abundance of mosquito larvae in relation to breeding habitats.
Figure 6: Abundance of mosquito larvae in relation to breeding habitats.
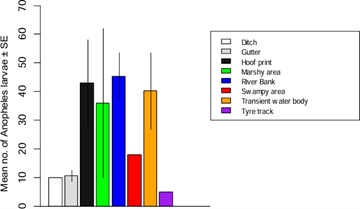 Figure 7: Abundance of Anopheles larvae in relation to habitat types.
Figure 7: Abundance of Anopheles larvae in relation to habitat types.
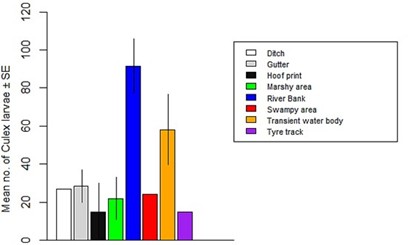 Figure 8: Abundance of Culex larvae in relation to breeding habitats.
Figure 8: Abundance of Culex larvae in relation to breeding habitats.
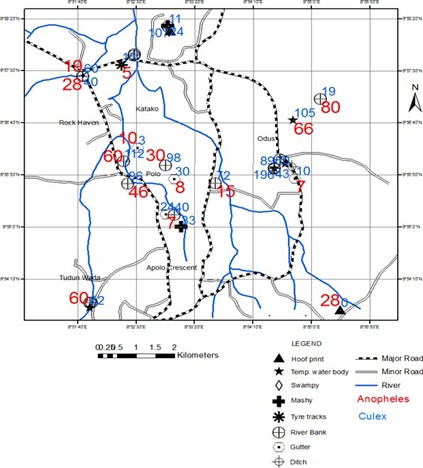 Figure 9: The density map of mosquito species and breeding habitats survey using GPS.
Figure 9: The density map of mosquito species and breeding habitats survey using GPS.
Discussion
The collection of 3,009 mosquito larvae, representing 2 genera and 2 species (Table 1), highlights the impact of unplanned urban expansion on mosquito breeding conditions in Jos North LGA. The lack of infrastructural development has created an environment conducive to mosquito breeding. These findings are consistent with previous research by Klinkenberg et al. [16], which demonstrated that unplanned urban expansion can lead to increased mosquito breeding sites. Thestudy’s results suggest that mosquito breeding habitats in Jos Northaremore prevalent in underdeveloped or poorly planned areas, characterized by high household and population density. These areas include slums and urban business districts. The non-significant difference in mosquito larvae abundance across locations (Figure 3) can be attributed to intensive irrigation farming in areas like Russau, which had the highest number of larvae collected. This finding is supported by Killeen [17], who linked high larvae abundance to agricultural activities. The significant difference in Anopheles larvae abundance across locations (Figure 4), with Rafin Karfe having the highest abundance, is consistent with the findings of Odikamnoro et al. [18]. However, the comparison of Culex larvae abundance showed no significant difference (Figure 5), with Russau having the highest abundance. This finding is in line with the work of Amerasinghe et al. [19], who reported high abundance of Culex, suggesting that the species is an indiscriminate breeder. The distribution of mosquito larvae across breeding habitats showed a significant difference (Figure 6). The highest abundance of mosquito larvae was collected from riverbank breeding habitats, which can be attributed to habitat preferences of the immature stages. These habitats, whether natural or man-made, temporary or permanent [20], provide ideal conditions for mosquito breeding. The high abundance of Anopheles larvae collected from riverbank breeding habitats can be attributed to habitat permanency, canopy cover, emergent plant cover, and wastewater [11]. Mutuku et al. [21] also found that Anopheles larvae prefer small habitats like hoof prints, temporary water bodies, and riverbanks. Similarly, high abundance of Culex larvae was collected from riverbank breeding habitats, consistent with the findings of Okiwelu and Noutcha [22], who recorded high numbers of Cx. quinquefasciatus larvae at stagnant polluted water.
Conclusion
This study investigated the spatial distribution and abundance of mosquito larvae in Jos North Local Government Area, Plateau State, Nigeria. The results revealed that Culex quinquefasciatus and Anopheles gambiae were the dominant species, with a significant difference in their abundance across different breeding habitats. Riverbank breeding habitats were found to be the most conducive for mosquito breeding, with high abundance of both species. The study’s findings highlight the need for effective mosquito control measures, particularly in areas with high household and population density.
Recommendations
To effectively manage mosquito populations and reduce disease transmission, we recommend the following
Strategies
- Implement Integrated Vector Management (IVM) incorporating environmental management, larval control, and adult mosquito control.
- Modify breeding habitats through proper drainage, removal of stagnant water, and vegetation
- Educate the public on mosquito control importance, particularly in high-risk
- Conduct regular surveillance to monitor mosquito populations and identify high-risk
- Foster collaboration among local government, health authorities, and stakeholders to develop effective control strategies.
- Pursue further research on environmental factors influencing mosquito breeding to inform evidence-based control measures.
References
1. Washno, R. K., & Wood, B. L. (1994). Application of remote sensing to vector arthropod surveillance and control. Am. J. Trop. Med. Hyg.50 (Suppl):134-144.
2. Dale, P. E., Ritchie, S. A., Territo, B. M., Morris, C. D., Muhar, A., & Kay, B.H. (1998). An overview of remote sensing and GIS for surveillance of mosquito vector habitats and risk assessment. J. Vector. Ecol., 23, 54-61
3. Arau jo, J. R., Ferreira, E., & De-Abreu. F. (2008). Revisaosistematicasobreestudos de espacializaçao da dengue no Brasil. Revista Brasileira deEpidemiologia 11, 696–708.
4. Foley, D. H., Rueda, L., & Wilkerson, M. (2007). Insight into global mosquito biogeography from country species records. J. of Med. Ento.44, 554–567.
5. Tauil, P., & Fontes C. J. (2009). Malaria, Doençastransmitidas e causadasporartropodes. Sao Paulo., 209-228.
6. Fang, J. (2010). A world without mosquitoes. J. of Ecol., 466(8), 432–434.
7. Forattini, O. P. (1996). Culicidologiamedica: princípiosgerais, morfologiaeglossariotaxonomico. SaoPaulo, Edusp, 1, 548549.
8. Honorio, N. A., Nogueira, C. T., Codeço, M. S., Carvalho, O. G. Cruz, M. S., & Pinel, R. (2009). Spatial evaluation and modeling of Dengue seroprevalence and vector density in Rio de Janeiro, Brazil. Neglected Tropical Diseases 3,1–11.
9. Cordeiro, R., Donalisio, V. R., Andrade, A. C., Mafra, L. B., & Nucci, J. C. (2011). Spatial distribution of the risk of dengue fever in southeast Brazil, 2006-2007. BMC Public Health 11, 355-366.
10. Barbosa, G. L., & Lourenço, R. W. (2010). Analise da distribuiçaoespaçotemporal de dengue e da infestaçaolarvaria no município de Tupa, estado de Sao Paulo.Revista da Sociedade. Brasileira de Medicina Tropical, 43, 145–151.
11. World Health Organization (2006). Malaria vector control and personal protection. Technical report series No. 936 World Health Organization Geneva.
12. Townshend, J., Mitchell, C. J., & Woodzick, T. L. (2000). Beware of per pixel characterization of land cover. Remote. Sensing.3(4): 839-843.
13. Harbach, R. E. (1985). Pictorial keys to the genera of mosquitoes, subgenera of Culex and the species of Culex occurring in Southwestern Asia and Egypt, with a note on the subgeneric placement of Culex deserticola (Diptera: Culicidae). Mosq.Syst., 17 (2), 83–107.
14. Harbach, R. E. (1988). Mosquitoes of the subgenus Culex in Southwestern Asia and Egypt (Diptera: Culicidae). Contr. Am.Ento. Inst., 24 (1), 1–240.
15. Glick, J.I. (1992). Illustrated key to the female Anopheles of Southwestern Asia and Egypt (Diptera: Culicidae). Mosq. Syst.,24, 125-153.
16. Klinkenberg, E., McCall, P., Wilson, M. D., Amerasinghe, F. P., & Donnelly, M. J., (2008). Impact of urban agriculture on malaria vectors in Accra, Ghana. Malaria Journal, 7. 151.
17. Killeen. (2002). Vector borne disease problems in rapid urbanization. Geneva Switzerland: WHO. Bull., 57.
18. Odikamnoro, O.O., Uhua, C.A., & Ibiam, G.A. (2012). A survey of mosquito species and implication for malaria transmission in Abakaliki, Ebonyi, State, Nigeria. Journal of Pharmacy and Biological Sciences, 3, 10-12.
19. Amerasinghe, F., Ariyasena, T., & Posa, M. (1990). Larval survey of surface water-breeding mosquitoes during irrigation development in the Mahaweli Project. Sri Lanka. J Med Entomol., 27, 789–802.
20. Bruce- Chwatt, L. J. (1991). Essential Malariology. Heinemann Medical Books Ltd. London. 462.
21. Mutuku, M. F., Alaii. J. A, Bayoh, M. N, Gimning, J. E Vulule, J. M, Walker, E. D Kabiru, E., & Hawley, W.A (2006). Distribution, description and local knowledge of larval habitat of Anopheles gambiae sensulato in a village in Western Kenya. American Journal of Tropical Medicine and Hygiene, 74: 44-53.
22. Okiwelu, S. N., & Noutcha, M. A. E. (2012). Breeding sites of Culex quinquefasciatus (Say) during the rainy season in rural lowland rainforest, Rivers State, Nigeria. Public Health Research, 2(4), 64-68.
This article licensed under the Creative Commons Attribution 4.0 International License CC-BY 4.0., which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are properly credited.
